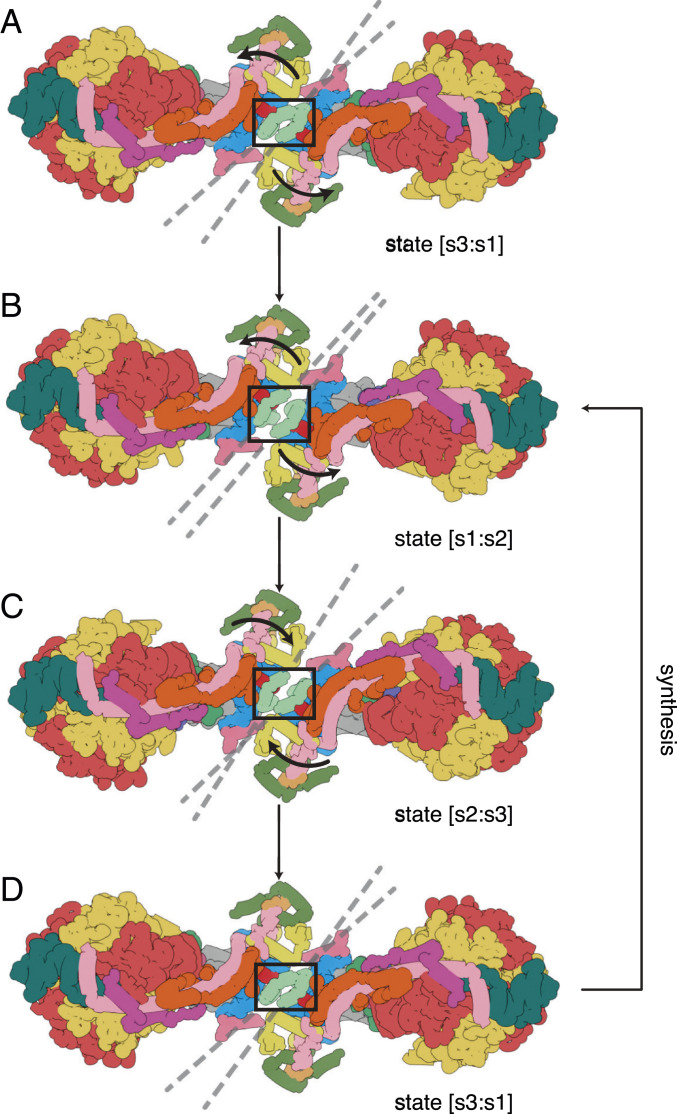
Fig. 2.
Pivoting of the membrane domains of bovine ATP synthase about the matrix contact between j subunits during ATP synthesis. Subunit j is sea-foam green inside the black box. For colors of other subunits, see Fig. 1 legend. (A) The state [s3:s1] dimer. Dashed lines indicate the central axis of amphipathic α-helices jH1 (residues 1–19), which lie in the plane of the matrix leaflet of the membrane. During the synthetic rotary cycle, each monomer progresses from state 1 to state 2 to state 3 and so forth. (B) Rotation of the membrane domain about the contact point in jH1 during the transition from state [s3:s1] to state [s1:s2], with accompanying displacement of subunits e, f, and g, and the transmembrane α-helices bH2 (residues 33–47) and bH3 (residues 55–73), moving the wedge outwards or inwards as indicated by the arrows. The membrane extrinsic F1 domains remain approximately stationary, and the rocking motion associated with the asymmetry of the central stalk is transmitted to the membrane domain via the universal joint between N- and C-terminal domains of the OSCP in the PS (2). (C) A similar pivoting motion occurs in the transition from state [s1:s2] to state [s2:s3]. (D) Completion of the rotary cycle by the transition from state [s3:s1] to state [s1:s2]. The synthetic rotary cycle continues via B as indicated by the arrow on the right. The scheme was constructed with composite atomic models of monomeric bovine ATP synthase, which were rigid body fitted into the consensus dimer structures produced according to SI Appendix, Scheme S1. This figure relates to Movie S3. In both this figure and Movie S3, side chains have been removed and secondary structure elements have been dilated to produce the diagrammatic nature of the figure, which is inferred from lower resolution structures of the intact dimeric complex.