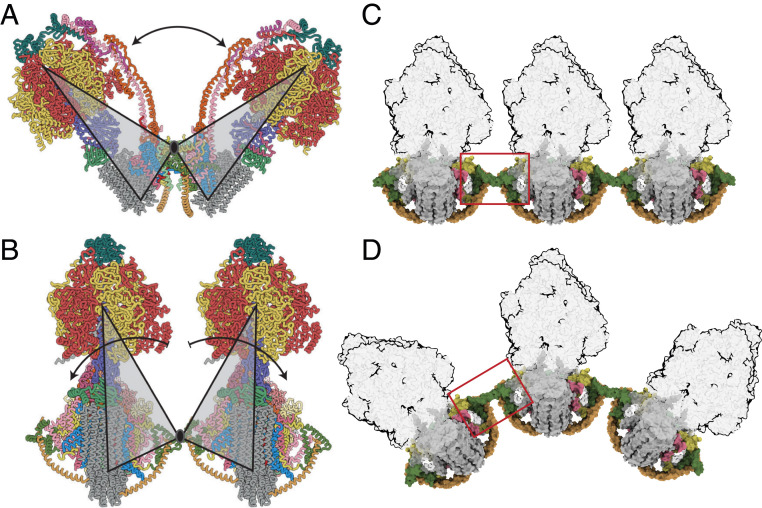
Fig. 5.
Accommodation of the dimeric ATP synthase to the membrane curvature in mitochondrial cristae along the axis of oligomerization via flexible interdimer contacts. (A) A view, orthogonal to the plane of the IMM, of the bovine ATP synthase dimer in state [s1:s1]. Gray triangles represent the monomers, moving back and forth as rigid bodies by rotation about their point of contact in j subunits; the rotatory axes of both monomers are aligned in the same plane, and the arrow indicates the range of angles between rotatory axes. (B) Side view, parallel to the plane of the IMM, of two monomers in a tetrameric arrangement of two adjacent dimers formed via contacts between g subunits. The arrows indicate the independent courses of their catalytic domains by rotation about this point. (C and D) Similar view to B, with the F1 domains in silhouette illustrating how changes in the interface between dimers (red box) allow a curved ultra-structure to develop along the apices of the cristae. For colors of subunits, see Fig. 1 legend.