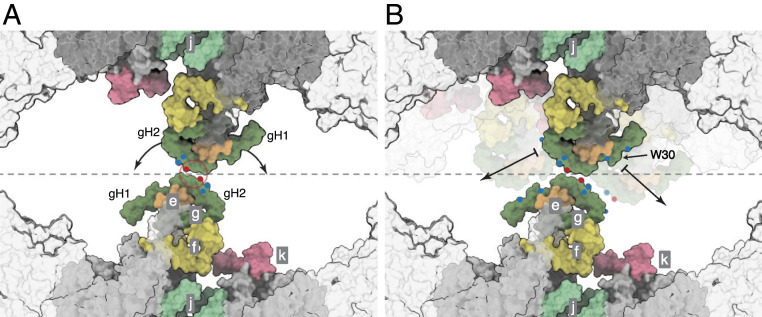
Fig. 6.
Close up of the dimer-dimer interface in a possible tetrameric bovine ATP synthase. The interactions shown as a molecular surface between g subunits in two adjacent dimers viewed from inside the mitochondrial matrix between the axes of the peripheral stalks, as in Fig. 7 B–G. Portions of the catalytic domains, situated above the viewing plane, are shown in gray transparency. The gray dashed line indicates the approximate tetramer interface, which is surrounded by lipids. (A) Rotation of the dimers relative to one another, as indicated by the arrows, about a contact point between the two g subunits, highlighted in the red circle. Key negative and positive polar residues are indicated by blue and red dots, respectively. (B) Contact dislocation of the interface between g subunits as indicated by the arrows. Possible positions of the translocated regions are shown in transparent underlay. Residue W30 might be involved in a hydrophobic “knobs-into-holes” interaction with regions of gH1 that are rich in leucine and isoleucine residues. Additional polar residues in gH1 and gH2 are indicated by colored dots. Subunits e, f, g, j, and k are khaki, straw yellow, forest green, sea-foam green, and pink, respectively. The c8 ring is gray.