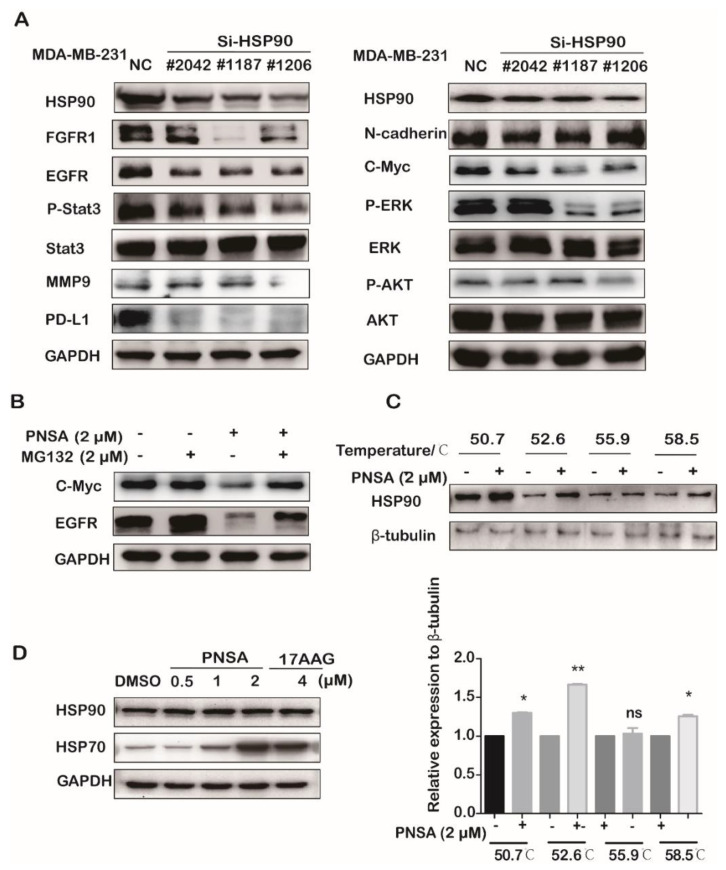
Figure 5.
PNSA inhibits the protein expressions and activations in the signaling pathway by targeting heat shock protein 90 (HSP90). (A) Knockdown of HSP90 causes changes in protein expressions and activations. MDA-MB-231 cell line transfected with Si-RNA or Si-NC were cultured in 6-well plates for 48 h. Protein expressions and activations were assessed by Western blotting. (B) PNSA degrades HSP90 clients EGFR and C-Myc via proteasome pathway. MDA-MB-231 cells were treated with PNSA or/and MG132 (protease inhibitor) for 12 h. Protein levels were assessed by Western blotting. GAPDH was used as a loading control. (C) Effect of PNSA on HSP90 protein stabilization. MDA-MB-231 cells were treated with 2 μM PNSA for 3 h before heated at different temperatures. Western blotting was used to determine the protein levels (top panel). The protein band density was quantified by normalization to β-Tubulin (bottom panel). (D) Effects of PNSA and 17-AAG on the expressions of HSP70 and HSP90. MDA-MB-231 cells were treated with PNSA (0.5, 1, 2 μM) or 4 μM 17-AAG for 12 h. Proteins levels were analyzed by Western blotting. GAPDH was used as a loading control. The bar graph represents the average ± SD of at least three independent experiments. * p < 0.05; ** p < 0.01; ns, not significant (relative to DMSO-treated cells).