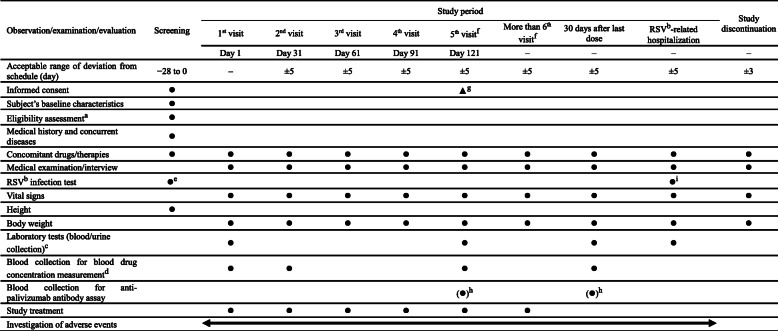
Table 3.
Study schedule
aThe eligibility of each subject will be assessed during the screening period
bRSV, respiratory syncytial virus
cHematology, blood biochemistry, and urinalysis
dBlood collection for blood drug concentration measurement must be performed prior to administration of palivizumab
eFor a RSV infection test during the screening period, a RSV rapid test (immunochromatography) kit will be used
fWhen the 5th, 6th, or 7th dose of palivizumab is administered, efficacy and safety measurements must be performed on Day 151, 181, or 211, respectively
gThe legally acceptable representative of the subject who requests continuation of treatment with palivizumab must provide written consent to the continued treatment prior to the administration of palivizumab at the 5th visit
hFirst, the subject’s health condition must be checked. Blood collection for laboratory tests should come first, followed by blood collection for anti-palivizumab antibody assay if possible
i RSV infection upon RSV-related hospitalization will be tested using the polymerase chain reaction (PCR) method