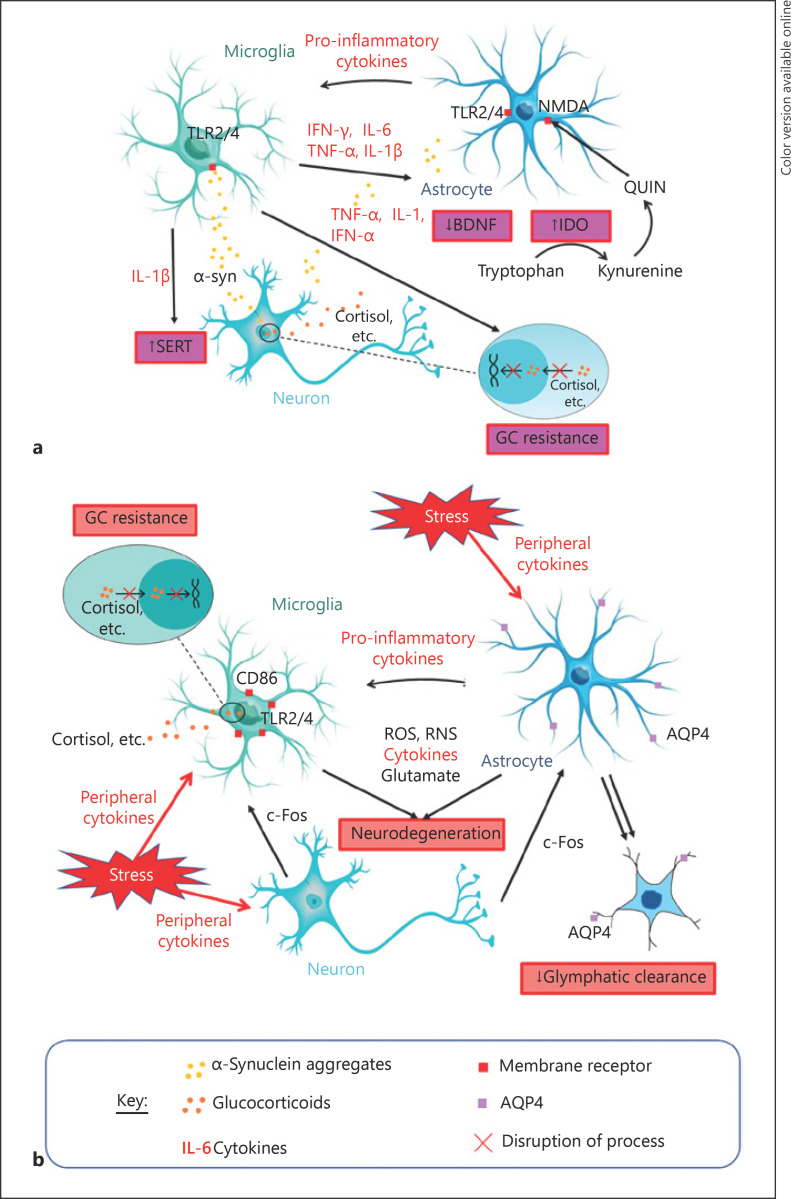
Fig. 2.
Summary of potential mechanisms linking PD and MDD. a MDD as a non-motor symptom of PD: oligomeric misfolded α-syn released by neurons is detected by microglia and astrocytes mainly via TLRs. Activated microglia release various pro-inflammatory markers, propagating neuroinflammation to other microglia and astrocytes and interacting with different processes that can contribute to the manifestation of depressive behaviours. IL-1β acts via IL-1β receptors and the p38 MAPK inflammatory pathway to increase serotonin reuptake from synapses through SERT, reducing synaptic serotonin levels to produce depressive behaviour [57]. TNF-α, IL-1, and IFN-α disrupt HPA axis function and cause GC resistance, which are hallmarks of depression. IFN-γ, IL-6, and TNF-α induce IDO, an enzyme that catabolizes the monoamine precursor tryptophan into kynurenine, which reduces capacity for monoamine synthesis by reducing concentrations of their precursor [35]. The kynurenine can be converted to an NMDA receptor agonist (QUIN) which stimulates increased glutamate release and reduces its uptake by astrocytes (an effect also observed due to increased circulating GCs), resulting in glutamate excitotoxicity [39, 40]. This excess glutamate combined with neuroinflammation reduce BDNF, impairing neural plasticity and subsequently contributing to depressive behaviour [39]. Inhibition of neural plasticity may also be caused by IL-1β through NF-κβ signalling activation [42]. b MDD as a prodrome or risk factor of PD: microglia and astrocytes respond to stress potentially via c-Fos signalling from neurons [51] and/or peripheral cytokines that have travelled past the BBB [57]. These activated glial cells propagate neuroinflammation to activate other glial cells, all of which may produce ROS, RNS, cytokines, and glutamate, which can lead to neurodegeneration through glutamate excitotoxicity and/or direct oxidative and nitrative damage [57, 61]. Stress also increases neuroinflammatory signalling proteins, including TLR4 and CD86, on microglial membranes, leading to the “priming” of microglia to subsequent pathogens to produce more vigorous α-syn-induced microglial activation in future [56]. Astrocyte activation results in pro-inflammatory cytokine secretion and impairs glymphatic clearance which further increases the risk of α-syn accumulation and PD pathology, possibly through reduced AQP4 distribution, structural atrophy of astrocytes, and/or physical reduction in astrocytes. The combination of these processes may increase the risk of PD as glymphatic disruption means that any misfolded fibrillar α-syn present is more likely to accumulate in the brain parenchyma. Following this, neuroinflammatory responses are able to do more significant damage as the microglia being activated have been primed [52, 69, 75, 76]. AQP, aquaporin; BDNF, brain-derived neurotrophic factor; CD, cluster of differentiation; GC, glucocorticoid; IDO, indoleamine 2,3-dioxygenase; IFN, interferon; IL, interleukin; NMDA, N-methyl-D-aspartate; QUIN, quinolinic acid; ROS, reactive oxygen species; RNS, reactive nitrogen species; SERT, serotonin transporter; TLR, toll-like receptor; TNF, tumour necrosis factor; α-syn, α-synuclein; MDD, major depressive disorder; PD, Parkinson's disease; MAPK, mitogen-activated protein kinase; HPA, hypothalamic-pituitary-adrenal; BBB, blood-brain barrier.