Abstract
Cancer-related fatigue has been related to circadian disruptions and lower levels of sleep quality. However, it is unknown whether the circadian phase, which is associated with chronotype and timing of sleep, is related to fatigue after cancer. The aims of this study were to investigate the associations between (1) chronotype and cancer-related fatigue and (2) sleep quality and cancer-related fatigue. In this cross-sectional questionnaire study, 458 (non-)Hodgkin lymphoma survivors (n = 231 female, mean age 49.7 years) completed a Visual Analogue Scale for fatigue (VAS-fatigue) from 0 (no fatigue) to 10 (worst imaginable fatigue), the Munich Chronotype Questionnaire (MCTQ), and the Pittsburgh Sleep Quality Index (PSQI) between October 2018 and July 2019. A hierarchical linear regression analysis was used to evaluate the associations between the dependent variable fatigue and chronotype (based on early, intermediate, or late average midsleep) in Model 1, and fatigue and sleep quality in Model 2. The results showed no indications for an association between chronotype and fatigue (all p values ≥ 0.50). There were associations between two (out of seven) aspects of sleep quality and fatigue: subjective sleep quality (p < 0.001) and daily dysfunctioning (p < 0.001). Therefore, it is more likely that fatigue is associated with self-reported sleep quality rather than with chronotype. However, experimental studies with objective, physiological data on circadian phase and sleep quality are necessary to confirm the conclusions of this cross-sectional study.
Keywords: circadian rhythms, fatigue, neoplasms, sleep, survivorship
Background
Cancer-related fatigue is one of the most frequently reported complaints in cancer survivors (Mortimer et al., 2010) with a prevalence between 25% and 60% in (non-)Hodgkin lymphoma (HL) survivors (Oerlemans et al., 2013; Daniëls et al., 2014). The National Comprehensive Cancer Network (NCCN) defined cancer-related fatigue as “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (Mock et al., 2000). The etiology of fatigue after cancer is still unknown but it is likely that multiple factors ranging from cognitive, emotional, psychosocial, and somatic factors are involved (O’Higgins et al., 2018).
One proposed underlying cancer-related fatigue mechanism is circadian rhythm disruption. Several studies, based on objective and subjective measurements, showed an association between circadian disruptions and fatigue, sleep disturbances, depression, and cognitive impairment (Rich, 2007; Payne, 2011; Innominato et al., 2014). These circadian disruptions in patients treated for cancer include a smaller amplitude of the rest-activity patterns (i.e., more sleep disruptions during the night and less activity during the day) and a flatter slope of the circadian rhythm of cortisol. This dampened rest-activity pattern was correlated with higher levels of fatigue in patients with metastatic colorectal cancer (Mormont and Waterhouse, 2002). In addition, a flatter cortisol slope was found for individuals with fatigue after cancer (Schrepf et al., 2013; Schmidt et al., 2016).
Yet, these results do not provide insight into the timing (i.e. phase advanced or phase delayed) of the circadian rhythm and its association with fatigue after cancer. Chronotype can be used as a marker of the circadian phase and is based on the timing of the sleep-wake cycle (Roenneberg et al., 2019). It is defined as the midpoint between sleep onset and awakening on days when no alarm clock is used. Individual differences between chronotypes exist due to genetic variance, age, and environment (Roenneberg et al., 2019). Some individuals are more prone to be active in the morning, so-called “larks,” and some individuals work better in the evening, so-called “owls.” Several studies showed an association between later chronotypes (the owls) and negative health outcomes like depression (Levandovski et al., 2011), bipolar disorders (Caruso et al., 2020), obesity (Soreca et al., 2009), and seasonal affective disorder (Lewy et al., 2006). It is also shown that a later chronotype is related to fatigue in individuals with irritable bowel symptoms (Chrobak et al., 2018) and students (Martin et al., 2012). However, the causal relationship between chronotype and negative health outcomes remains unclear. One explanation might be the misalignment between the circadian clock and social obligations. For example, extremely late evening types experience a need to sleep around 0300 h in the morning. Yet, society obligates these individuals to set an early alarm, creating a sleep debt during the week. This phenomenon is also known as a social jet lag (Roenneberg et al., 2019).
On the contrary, circadian rhythm disruptions have been associated with lower levels of sleep quality in cancer patients (Ancoli-Israel et al., 2006). Sleep disturbances and sleep disorders are well studied in patients treated for cancer and prevalence rates up to 62% have been reported in patients with cancer compared with 30% in healthy volunteers. This prevalence remains higher in patients treated for cancer compared with healthy volunteers up to 18 months after diagnosis (Anderson et al., 2003). Several studies showed an association between poorer sleep quality and increased levels of fatigue in patients treated for cancer (Coles et al., 2018; Fortmann et al., 2018). Related to chronotype, several studies in other populations showed that evening types had worse sleep quality (Soreca et al., 2009; Martin et al., 2012; Caruso et al., 2020).
The reason to study the associations between chronotype and cancer-related fatigue and sleep quality and cancer-related fatigue is three-fold. First, studies in other populations showed associations between eveningness and fatigue (Soreca et al., 2009; Martin et al., 2012; Chrobak et al., 2018; Caruso et al., 2020) suggesting that this association might also be present in other populations. Second, it provides information on the potential working mechanism of morning light therapy as a treatment for fatigue after cancer (Ancoli-Israel et al., 2012; Redd et al., 2014; Johnson et al., 2017). As morning light therapy advances the circadian phase, which is associated with chronotype (Lewy et al., 1998), a later chronotype might be associated with fatigue. Alternatively, light therapy has been shown to improve sleep quality (Wams et al., 2017), which might be the working mechanism of light therapy for decreasing fatigue. Third, a description of chronotypes in cancer survivors with cancer-related fatigue provides information on the optimal timing of light therapy (e.g., when cancer survivors with fatigue are more often morning types, morning light therapy will shift them even earlier which is not desired). Therefore, the current study aimed to explore the associations between (1) chronotype and fatigue after cancer and (2) sleep quality and fatigue after cancer. It was expected that survivors with moderate to severe fatigue would show a delayed chronotype, that is, being an evening type, and report poorer sleep quality compared with survivors with no to mild fatigue. If this is the case, light therapy in the morning might decrease symptoms of fatigue after cancer.
Methods
Study Participants
For this cross-sectional study, individuals were invited to participate in the study if they met the following inclusion criteria: (1) diagnosis of HL or diffuse large B-cell lymphoma (DLBCL) at least 2 years ago, (2) no treatment for cancer in the past 12 months, and (3) sufficient knowledge of the Dutch language. Individuals were excluded if they reported to work in nightshifts.
Procedure
The hematologist or radiation oncologist in seven hospitals in the Netherlands (Admiraal de Ruyter hospital, Albert Schweitzer hospital, Amsterdam UMC [location AMC], Erasmus MC, Haga hospital, Leiden University Medical Center, University Medical Center Utrecht) identified eligible participants. Based on the inclusion criteria, a total number of 761 eligible survivors were identified. These individuals received an information package from their treating physician, including an invitation letter, a patient information letter with informed consent, our questionnaire, and a return envelope. The package also included additional information on a clinical trial testing light therapy as a treatment for fatigue after cancer (SPARKLE study; Starreveld et al., 2018). Participants with fatigue could request more information about this clinical trial via a response card. All participants returned a signed informed consent form and the completed questionnaire by mail to the study coordinator.
In addition, Hematon (the patient organization of lymphoma patients in the Netherlands) included a message in their monthly newsletter to their members (>4300 members) to inform them about the study. This message included a link to an online version of the questionnaire. Only those responders who expressed interest and left contact details were contacted for further screening for eligibility in the SPARKLE study. No contact details were available for responders who completed the survey but were not interested in the SPARKLE study.
Study procedures conformed to the Declaration of Helsinki. Ethical approval for the study was obtained from the Institutional Review board of the Netherlands Cancer Institute (under number NL61017.031.17). Questionnaires were completed between October 2018 and July 2019.
Measurements
Sociodemographic data included self-reported age, gender, education, marital status, living situation, and work status. Clinical data, including diagnosis, date of diagnosis (month and year), treatment history, height, and weight were also obtained via self-report. Height and weight were used to calculate body mass index (BMI), which was categorized into normal (18.5-24.9), overweight (25-30), and obese (> 30). There were two underweight cases (BMI < 18.5) who were included in the normal category. Comorbidities were assessed by an adapted version of the Self-administered Comorbidity Measure (Sangha et al., 2003).
The Munich Chronotype Questionnaire (MCTQ; Roenneberg et al., 2003) was used to measure sleep timing on work days and work-free days. Fourteen items cover bedtime, sleep time, sleep latency, wake time, sleep inertia, alarm clock use, and light exposure on workdays and work-free days. Based on the completed items of the MCTQ, sleep onset was calculated as the sum of the time to get ready to fall asleep (preparation time) and the minutes needed to fall asleep (sleep latency). Sleep duration was calculated as the difference between sleep onset and sleep offset. Total time in bed was calculated as the difference between bedtime and the time someone gets out of bed. Average midsleep (aMS) was calculated as the midpoint between sleep onset and sleep offset on all days of the week. aMS was used as an indicator for chronotype (Kantermann and Burgess, 2017) and categorized in five categories: moderate to extremely early (aMS before 0200 h), slightly early (aMS between 0200 and 0300 h), intermediate (aMS between 0300 and 0400 h), slightly late (aMS between 0400 and 0500 h), and extremely late (aMS of 0500 h or later) aMS based on the chronotype distribution in the MCTQ population (Roenneberg et al., 2019). Social jetlag was calculated as the difference of the midpoint between sleep onset and sleep offset on workdays and free days. Employment was categorized in three categories: unemployed (0 workdays), employed part-time (1-4 workdays), and employed full-time (4 or more workdays).
A VAS scale ranging from 0 (no fatigue) to 10 (worst imaginable fatigue) was used to assess fatigue. Based on the VAS score, fatigue was categorized as no to mild fatigue (VAS scores ≤ 3), moderate fatigue (VAS range from 4 to 7), or severe fatigue (VAS ≥ 7) (Oldenmenger et al., 2013).
Fatigue was described in more detail by the general fatigue score of the Multidimensional Fatigue Inventory (MFI; Smets et al., 1995). Originally, the MFI measures five domains of fatigue (general fatigue, mental fatigue, physical fatigue, reduced motivation, and reduced activity) but we recently showed that this factor structure is questionable. The general fatigue subscale is the most stable measurement for fatigue and was therefore included in our analysis. In addition, the relationship between fatigue and cancer or its treatment was assessed by asking the following question: “Have you experienced persistent fatigue since the diagnosis of and/or treatment for cancer?” which was answered with “yes” or “no.”
The Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) was included to assess sleep quality. This 19-item questionnaire measures various aspects of sleep patterns and sleep quality including seven subscales: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disruptions, use of sleep medication, and daily dysfunctioning. Questions 1 to 4 cover bedtime, sleep inertia, get-up time, and sleep duration and were derived from the MCTQ to avoid repetition in the questionnaire. Scores on the subscales range between 0 (no difficulty) and 3 (severe difficulty) and were included as categorical variables (score 0, 1, 2, or 3) in the hierarchical regression model. The complete score ranges between 0 (good sleep quality) and 21 (worse sleep quality) and was used for descriptive purposes only.
Statistical Analyses
Sociodemographic, clinical, fatigue, and sleep characteristics of the study population were described using descriptive statistics for the entire sample and, separately, for survivors with no to mild, moderate, or severe fatigue after cancer. Differences between groups for continuous variables were tested with one-way analyses of variance (ANOVAs) and Bonferroni post hoc procedures. For non-normal distributions or unequal variances, Kruskal-Wallis tests were used. Chi-square tests were used to study group differences for categorical variables. Fisher’s Exact tests were used when the cross table included one or more cells with less than five observations. Bonferroni corrected p values were used to correct for multiple testing (see footnote under Table 2).
Table 2.
Mean (SD) bedtime and sleep quality information for all survivors and for survivors with no, moderate, or severe fatigue separately.
| Total (N = 458) |
No Fatigue (n = 134) |
Moderate Fatigue (n = 171) |
Severe Fatigue (n = 133) |
p Value | Post Hoc Comparison | Missing (%) | |
|---|---|---|---|---|---|---|---|
| Bed timea | |||||||
| Basic variables | |||||||
| I go to bed at . . . o’clock | 23:03 (0:59) | 23:23 (0:59) | 22:59 (0:58) | 22:48 (0:57) | <0.001* | N > M, S | 0.9 |
| I get ready to fall asleep at . . . o’clock | 23:25 (0:55) | 23:37 (0:56) | 23:26 (0:50) | 23:14 (0:57) | 0.004* | N > S | 1.1 |
| I need . . . minutes to fall asleepb | 17 (22) | 11 (13) | 17 (19) | 24 (31) | <0.001* | N, M < S | 1.7 |
| I wake up at . . . o’clockb | 7:43 (1:25) | 7:49 (1:10) | 7:39 (1:24) | 7:45 (1:36) | 0.86 | 1.5 | |
| After . . . minutes I get up | 32 (56) | 22 (32) | 33 (51) | 39 (60) | 0.006 | 2.6 | |
| Hours spent outsideb | 2:38 (1:49) | 3:17 (2:14) | 2:20 (1:29) | 2:17 (1:34) | <0.001* | N > M, S | 10.9 |
| Calculated variables | |||||||
| Sleep onset | 23:42 (0:59) | 23:48 (0:59) | 23:43 (0:54) | 23:36 (1:06) | 0.29 | 1.3 | |
| Sleep durationb | 8:00 (1:28) | 8:01 (1:07) | 7:55 (1:32) | 8:09 (1:41) | 0.05 | 2.0 | |
| Total time in bed | 9:10 (1:18) | 8:48 (1:03) | 9:12 (1:21) | 9:35 (1:21) | <0.001* | N < M < S | 1.7 |
| Average midsleep | 3:19 (0:51) | 3:18 (0:48) | 3:19 (0:47) | 3:22 (0:58) | 0.76 | 3.3 | |
| Moderate/extreme early, n (%)c | 21 (5) | 4 (3) | 7 (4) | 9 (7) | 0.74 | 3.3 | |
| Slightly early, n (%) | 124 (27) | 42 (32) | 44 (27) | 31 (24) | |||
| Intermediate, n (%) | 211 (46) | 64 (48) | 78 (48) | 62 (48) | |||
| Slightly late, n (%) | 75 (16) | 20 (15) | 30 (19) | 23 (18) | |||
| Moderate/extreme late, n (%) | 12 (3) | 3 (2) | 3 (2) | 5 (4) | |||
| Social jetlag | 0:44 (0:43) | 0:51 (0:45) | 0:41 (0:41) | 0:40 (0:44) | 0.06 | 2.2 | |
| Sleep qualitya | |||||||
| Subjective sleep qualityb | 1.1 (0.7) | 0.6 (0.6) | 1.2 (0.6) | 1.4 (0.8) | <0.001* | N < M < S | 0.4 |
| Sleep latency | 1.0 (0.9) | 0.5 (0.8) | 1.0 (0.9) | 1.3 (1.0) | <0.001* | N < M < S | 1.7 |
| Sleep duration | 0.4 (0.8) | 0.5 (0.8) | 0.4 (0.8) | 0.4 (0.8) | 0.51 | 2.0 | |
| Sleep efficiencyb | 0.4 (0.7) | 0.1 (0.4) | 0.5 (0.9) | 0.4 (0.8) | <0.001* | N < M, S | 2.0 |
| Sleep disruptionsb | 1.3 (0.6) | 1.1 (0.5) | 1.3 (0.5) | 1.5 (0.7) | <0.001* | N < M < S | 6.8 |
| Sleep medicationb | 0.3 (0.8) | 0.1 (0.4) | 0.3 (0.8) | 0.4 (0.9) | <0.001* | N < M, S | 0 |
| Daily dysfunctioningb | 1.1 (0.8) | 0.4 (0.6) | 1.2 (0.7) | 1.6 (0.8) | <0.001* | N < M < S | 0.9 |
| Total scoreb | 5.4 (3.3) | 3.4 (2.2) | 5.8 (3.2) | 6.8 (3.4) | <0.001* | N < M < S | 9.0 |
Abbreviations: N = no fatigue; M = moderate fatigue; S = severe fatigue. The 24-h clock notation is used for questions regarding time (22:30 is half past 2200 h) and duration (0:30 is 30 min, that is, 0.5 h).
Bonferroni corrected p value of 0.004 (0.05/12) or less was considered to be statistically significant for bedtime variables. A Bonferroni corrected p value of 0.006 (0.05/8) or less was considered to be statistically significant for sleep quality variables.
Kruskal-Wallis test reported.
Fisher’s Exact Test reported.
p < 0.004 for bedtime variables or p < 0.006 for sleep quality variables.
Pearson correlation analysis were used to test bivariate associations between chronotype, sleep quality, and fatigue. A hierarchical linear regression analysis was used to evaluate the association between fatigue and aMS and fatigue and sleep quality. In the first model, the continuous score of the VAS-fatigue was used as the dependent variable and aMS as independent variable. For the regression analyses, we combined the categories “slightly morning” and “moderate to extreme morning” into morning type and “slightly evening” and “moderate to extreme evening” into evening type to reduce potential bias by the small number of extreme types. In the second model, the seven sleep quality subscales of the PSQI were added as categorical independent variables to the first model. Both models included age (years), time since diagnosis (years), and comorbidities (number) as continuous factors and sex (male: yes/no), BMI (overweight: yes/no; obese: yes/no), marital status (married or living together: yes/no), education (college or university: yes/no), diagnosis (non-HL: yes/no), part-time employment (yes/no), and full-time employment (yes/no) as categorical factors to control for their effects on fatigue or chronotype (Roenneberg et al., 2019). There were no treatment variables included in the regression models because previous studies showed that treatment had no effect on fatigue scores in survivors of HL (Kreissl et al., 2016). Bonferroni corrected p values were used to correct for multiple testing (see footnote under Table 3).
Table 3.
Linear model of independent variables on the continuous value of the VAS-fatigue with imputed data (N = 458).
| Model 1a |
Model 2a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | (SE) | 95% CI | p | B | (SE) | 95% CI | p | |||
| Lower | Upper | Lower | Upper | |||||||
| Constant | 7.88 | 0.65 | 6.59 | 9.16 | 3.72 | 0.69 | 2.38 | 5.07 | ||
| Intermediate aMS | 0.14 | 0.25 | −0.35 | 0.63 | 0.58 | −0.02 | 0.21 | −0.43 | 0.40 | 0.94 |
| Late aMS | −0.01 | 0.33 | −0.66 | 0.63 | 0.97 | −0.34 | 0.29 | −0.92 | 0.23 | 0.24 |
| Age | −0.06 | 0.01 | −0.08 | −0.04 | <0.001* | −0.03 | 0.01 | −0.04 | −0.01 | 0.01 |
| Male | −0.64 | 0.24 | −1.12 | 0.16 | 0.01 | −0.46 | 0.21 | −0.87 | −.06 | 0.03 |
| BMI: Overweight | 0.34 | 0.25 | −0.15 | 0.83 | 0.17 | 0.26 | 0.20 | −0.14 | 0.65 | 0.21 |
| BMI: Obese | 0.35 | 0.33 | −0.31 | 1.00 | 0.30 | 0.18 | 0.27 | −0.36 | 0.72 | 0.51 |
| Married | −0.27 | 0.26 | −0.77 | 0.24 | 0.30 | 0.18 | 0.21 | −0.24 | 0.60 | 0.40 |
| College or university | 0.15 | 0.24 | −0.31 | 0.62 | 0.52 | −0.16 | 0.20 | −0.55 | 0.22 | 0.41 |
| NHL | 0.32 | 0.28 | −0.23 | 0.86 | 0.25 | 0.04 | 0.22 | −0.40 | 0.48 | 0.85 |
| Time since diagnosis | 0.00 | 0.01 | −0.03 | 0.03 | 0.99 | 0.00 | 0.01 | −0.02 | 0.02 | 0.72 |
| Comorbidities | 0.40 | 0.08 | 0.25 | 0.55 | <0.001* | 0.05 | 0.07 | −0.08 | 0.18 | 0.45 |
| Part-time employment | −0.94 | 0.35 | −1.62 | −0.25 | 0.01 | −0.49 | 0.30 | −1.07 | 0.10 | 0.10 |
| Full-time employment | −0.88 | 0.32 | −1.50 | −0.25 | 0.006 | −0.23 | 0.28 | −0.77 | 0.32 | 0.43 |
| Subjective Sleep Quality 1 | 0.89 | 0.26 | 0.38 | 1.39 | 0.001* | |||||
| Subjective Sleep Quality 2 | 1.47 | 0.35 | 0.78 | 2.15 | <0.001* | |||||
| Subjective Sleep Quality 3 | 2.80 | 0.68 | 1.47 | 4.14 | <0.001* | |||||
| Sleep Latency 1 | 0.19 | 0.23 | −0.27 | 0.64 | 0.42 | |||||
| Sleep Latency 2 | 0.43 | 0.30 | −0.15 | 1.01 | 0.14 | |||||
| Sleep Latency 3 | −0.08 | 0.46 | −0.84 | 0.99 | 0.87 | |||||
| Sleep Duration 1 | −0.26 | 0.25 | −0.76 | 0.23 | 0.30 | |||||
| Sleep Duration 2 | −1.00 | 0.40 | −1.78 | −0.21 | 0.01 | |||||
| Sleep Duration 3 | −1.08 | 0.61 | −2.28 | 0.13 | 0.08 | |||||
| Sleep Efficiency 1 | 0.02 | 0.29 | −0.54 | 0.58 | 0.95 | |||||
| Sleep Efficiency 2 | 1.00 | 0.48 | 0.05 | 1.95 | 0.04 | |||||
| Sleep Efficiency 3 | 1.14 | 0.71 | 0.25 | 2.53 | 0.11 | |||||
| Sleep Disruptions 1 | −0.50 | 0.40 | −1.29 | 0.29 | 0.21 | |||||
| Sleep Disruptions 2 | −0.50 | 0.46 | −1.40 | 0.39 | 0.27 | |||||
| Sleep Disruptions 3 | 0.42 | 0.79 | −1.14 | 1.97 | 0.60 | |||||
| Sleep Medication 1 | −0.03 | 0.60 | −1.21 | 1.15 | 0.96 | |||||
| Sleep Medication 2 | −0.06 | 0.55 | −1.13 | 1.02 | 0.92 | |||||
| Sleep Medication 3 | 0.42 | 0.40 | −0.37 | 1.22 | 0.30 | |||||
| Daily Dysfunctioning 1 | 2.15 | 0.23 | 1.70 | 2.60 | <0.001* | |||||
| Daily Dysfunctioning 2 | 3.09 | 0.28 | 2.53 | 3.64 | <0.001* | |||||
| Daily Dysfunctioning 3 | 3.28 | 0.50 | 2.31 | 4.26 | <0.001* | |||||
Abbreviations: CI = confidence interval; aMS = average midsleep; BMI = body mass index; NHL = non-Hodgkin lymphoma. Intermediate aMS: intermediate aMS (1) versus early aMS (0) and late aMS (0); Late aMS: late aMS (1) versus early aMS (0) and intermediate aMS (0); Age: included as continuous variables in years; Male: male (1) versus female (0); BMI overweight: BMI overweight (1) versus BMI healthy (0) and BMI obese (0); BMI obese: BMI obese (1) versus BMI healthy (0) and BMI overweight (0); Married: married or living together (1) versus single, widow or divorced (0); College or university: college or university (1) versus primary education, high school, or vocational education (0); NHL: non-Hodgkin lymphoma (1) versus Hodgkin lymphoma (0); Time since diagnosis: included as continuous variable in years; Comorbidities: included as continuous variable in number of self-reported comorbidities. Part-time employment: part-time employed (1) versus no employment (0) or full-time employment (0). Full-time employment: full-time employed (1) versus no employment (0) or part-time employment (0). Subjective sleep quality: reference category is good subjective sleep quality (0). Sleep latency: reference category is no problems (0). Sleep duration: reference category is more than 7 h (0). Sleep efficiency: reference category is more than 85% (0). Sleep disruptions: reference category is no disruptions (0). Sleep medication: reference category is no sleep medication (0). Daily dysfunctioning: reference category is no dysfunctioning (0).
For Model 1, a Bonferroni corrected p value of 0.0038 (0.05/13) was used. For Model 2, a Bonferroni corrected p value of 0.0015 (0.05/34) was used.
p < 0.0038 (Model 1) or < 0.0015 (Model 2).
Missing values in the 19 variables included in the hierarchical regression were imputed. First, single imputation was used on two items of the MCTQ based on the following imputation rules: (1) Missing “preparation time to go to sleep” was copied from “bedtime” and (2) “Sleep latency” was copied from “sleep latency” of the other day (work or free day) if available. After this single imputation, multiple imputation (Rubin, 1987) was used to create and analyze 10 multiply imputed datasets. Incomplete variables were imputed under fully conditional specifications, using the default settings of the Mice 3.7 package (van Buuren and Groothuis-Oudshoorn, 2011), in R version 3.6.1 (R Core Team, 2013). All 19 variables included in the regression were used in the imputation model as well as all auxiliary variables used to create the PSQI subscales and all MFI items. The parameters of substantive interest were estimated in each imputed dataset separately, and combined using Rubin’s rules. For comparison, we also performed the analysis on the subset of complete cases. All statistical analyses were performed using SPSS version 25.0 or R version 3.6.1.
Results
Participants
Of the 761 eligible participants who were invited through the hospitals, 430 returned a questionnaire on paper (response rate of 57%). Recruitment via the newsletter of the patient federation Hematon led to 91 online responses. In total, 521 questionnaires were returned. From the online reactions, 37 of the 91 participants completed less than 70% of the questionnaire and were excluded from analyses. Twenty-six participants were excluded from analyses due to shiftwork, leading to an analytic sample of 458 participants.
The mean age of the analytic sample was 49.7 years (SD = 12.3), with 231 females (50%). The majority (71%) was diagnosed with HL. The mean time since diagnosis was 12.0 years (SD = 9.7). Ninety-three percent of the participants received chemotherapy, 60% received radiotherapy, and 25% received other treatments. The majority (68%) reported at least one comorbidity. See Table 1 for more details.
Table 1.
Sociodemographic, clinical, and fatigue characteristics for all survivors and for survivors with no, moderate, or severe fatigue separately.
| No. (%) | p Value | Post Hoc | Missing (%) | ||||
|---|---|---|---|---|---|---|---|
| Total (N = 458) |
No Fatigue (n = 134) |
Moderate Fatigue (n = 171) |
Severe Fatigue (n = 133) |
||||
| Age in years | 0.002** | N > M, S | 1.5 | ||||
| M | 49.7 | 52.6 | 47.9 | 48.4 | |||
| SD | 12.3 | 11.7 | 12.0 | 12.9 | |||
| 20−35 years | 71 (16) | 14 (11) | 29 (18) | 27 (21) | 0.005** | ||
| 36−50 years | 147 (32) | 32 (24) | 66 (40) | 42 (32) | |||
| 51−65 years | 186 (41) | 67 (50) | 58 (35) | 52 (40) | |||
| 65−75 years | 47 (10) | 20 (15) | 13 (8) | 11 (8) | |||
| Sex | |||||||
| Female | 231 (50) | 47 (35) | 91 (55) | 85 (64) | <0.001*** | 1.1 | |
| Male | 222 (49) | 87 (65) | 76 (46) | 47 (36) | |||
| BMI (SD) | 0.12 | 2.2 | |||||
| M | 26.1 | 25.3 | 26.5 | 25.9 | |||
| SD | 4.6 | 4.3 | 4.8 | 4.5 | |||
| 16.5−25a | 207 (45) | 73 (56) | 71 (43) | 60 (46) | 0.25 | ||
| 25−30 | 171 (37) | 40 (31) | 67 (40) | 52 (39) | |||
| >30 | 90 (15) | 18 (14) | 28 (17) | 20 (15) | |||
| Living situation | |||||||
| Married | 340 (74) | 107 (80) | 132 (79) | 87 (67) | 0.02* | 1.5 | |
| Educationb | |||||||
| None/Primary education | 7 (2) | 1 (1) | 2 (1) | 4 (3) | 0.70 | 1.5 | |
| High school and vocational education | 229 (50) | 66 (50) | 86 (52) | 65 (50) | |||
| College and university | 215 (47) | 66 (50) | 79 (47) | 62 (47) | |||
| Number of working days | 0.001** | N > S | 1.1 | ||||
| M | 2.9 | 3.4 | 2.9 | 2.5 | |||
| SD | 2.1 | 2.1 | 2.1 | 2.2 | |||
| Employment status | |||||||
| Unemployed | 126 (29) | 28 (21) | 47 (28) | 51 (39) | 0.01 | 1.1 | |
| Employed part-time | 84 (19) | 23 (17) | 37 (22) | 24 (18) | |||
| Employed full-time | 223 (52) | 83 (62) | 83 (50) | 57 (43) | |||
| Diagnosisb | |||||||
| HL | 324 (71) | 92 (70) | 122 (72) | 94 (71) | 0.26 | 1.0 | |
| DLBCL | 74 (16) | 27 (21) | 28 (17) | 17 (13) | |||
| Aggressive NHL | 14 (3) | 1 (1) | 6 (4) | 6 (5) | |||
| Low grade NHL | 8 (2) | 1 (1) | 2 (1) | 5 (4) | |||
| NHL, unknown origin | 26 (6) | 10 (8) | 8 (5) | 7 (5) | |||
| Other | 8 (2) | 1 (1) | 3 (2) | 4 (3) | |||
| Time since diagnosis in years | 0.56 | 2.2 | |||||
| M | 12.0 | 11.6 | 12.5 | 11.3 | |||
| SD | 9.7 | 9.3 | 9.8 | 9.5 | |||
| 0−5 years | 126 (28) | 39 (30) | 44 (27) | 40 (30) | 0.95 | ||
| 6−15 years | 184 (40) | 53 (40) | 68 (41) | 54 (41) | |||
| > 15 years | 138 (30) | 40 (30) | 53 (32) | 38 (29) | |||
| Treatment | |||||||
| Chemotherapy | 424 (93) | 127 (96) | 155 (91) | 122 (92) | 0.32 | 0.4 | |
| Radiotherapy | 276 (60) | 84 (63) | 104 (61) | 76 (57) | 0.59 | 0.4 | |
| Other treatmentsc | 112 (25) | 33 (25) | 39 (23) | 35 (26) | 0.79 | 0.4 | |
| Self-reported comorbidities (in past 12 months) | |||||||
| 0 | 137 (30) | 59 (45) | 43 (26) | 30 (23) | <0.001*** | 2.8 | |
| 1 | 126 (28) | 38 (29) | 51 (31) | 34 (26) | |||
| ≥ 2 | 182 (40) | 35 (27) | 72 (43) | 65 (50) | |||
| Fatigue | |||||||
| General fatigue | <0.001*** | N < M < S | 0 | ||||
| M | 12.7 | 7.9 | 14.0 | 16.3 | |||
| SD | 4.7 | 3.2 | 3.4 | 2.9 | |||
| Cancer-related fatigue (yes) | 300 (66) | 28 (21) | 133 (79) | 127 (96) | <0.001*** | 1.1 | |
Abbreviations: N = no fatigue; M = moderate fatigue; S = severe fatigue; BMI = body mass index; HL = Hodgkin lymphoma; DLBCL = diffuse large B-cell lymphoma; NHL = non-Hodgkin lymphoma.
Two underweight cases (BMI between 16.5 and 18.5) were included in the normal BMI category.
Fisher’s Exact Test reported.
Other treatments include stem cell transplantation, surgery, immunotherapy, or wait and see.
p < 0.05. **p < 0.01. ***p < 0.001.
Fatigue
Based on the VAS-fatigue scale, 134 survivors (29%) reported to experience no to mild fatigue, 171 survivors (37%) reported moderate fatigue, and 133 (29%) reported severe fatigue since diagnosis or treatment for cancer (20 survivors [4%] did not complete the VAS-fatigue scale). General fatigue (MFI) and the proportion of individuals that report fatigue since cancer were higher in the moderately and severely fatigued group than in the no to mild fatigued group.
Bedtime Information
Bedtime information on free days per group is shown in Table 2 for the entire sample and, separately, for groups based on the VAS-fatigue. There were no differences for sleep onset, wake-up time, and sleep duration between groups. However, there was a significant difference in total time spent in bed, which was increased in survivors with severe fatigue (9 h 35 min) compared with survivors with moderate fatigue (9 h 12 min), who stayed longer in bed compared with survivors without fatigue (8 h 48 min). This difference in total time spent in bed can be explained by the finding that moderately and severely fatigued survivors had a statistically significant earlier bed time (36 and 25 min, respectively) compared with no-fatigued survivors and tended to have a longer sleep inertia. There were no differences in aMS between groups, probably explained by comparable sleep onset and wake-up times between groups.
Based on the aMS, 145 survivors (32%) were classified as morning types (M = 2:26; SD = 0:29), 211 survivors (46%) as intermediate types (M = 3:26; SD = 0:17), and 87 (19%) as evening types (M = 4:31; SD = 0:36). For 15 survivors (3%), aMS could not be calculated due to missing data.
Bedtime Information, Sleep Quality, and Fatigue
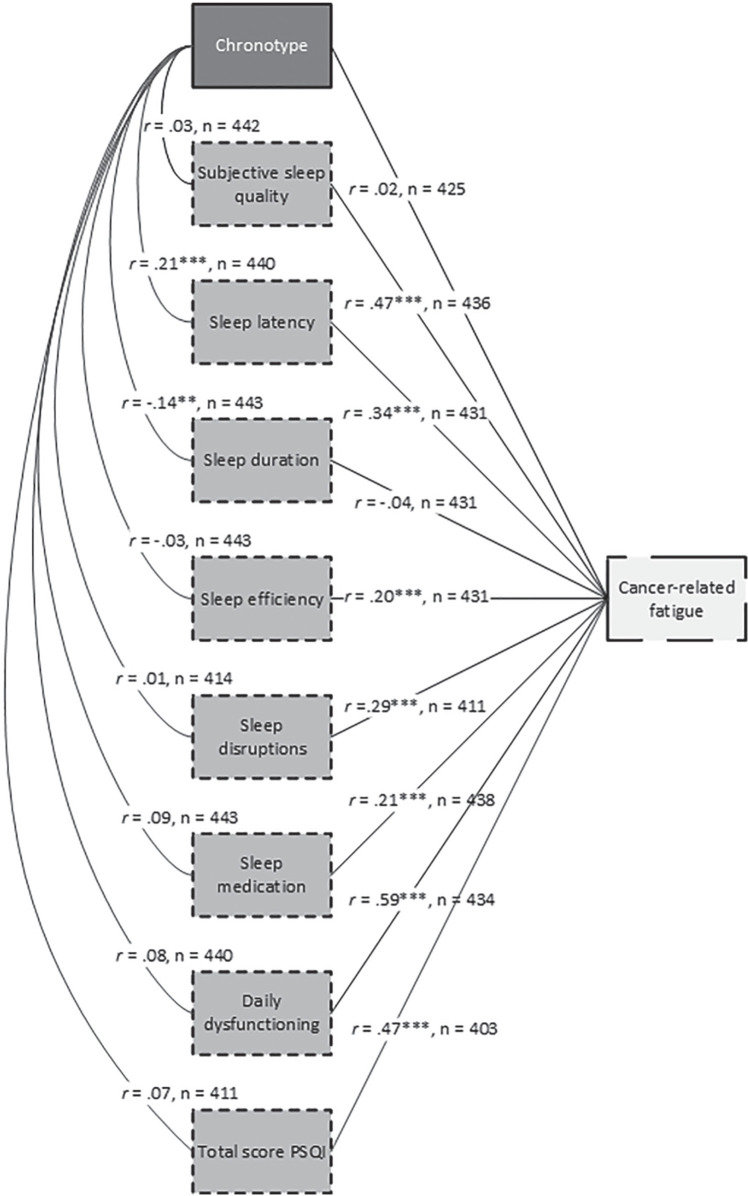
Figure 1 shows a schematic overview of bivariate Pearson correlations between chronotype, sleep quality, and fatigue. Chronotype was significantly associated with two aspects of sleep quality: sleep latency (r = 0.21; p < 0.001) and sleep duration (r = 0.14; p < 0.01). With the exception of chronotype (r = 0.02; p = 0.62) and sleep duration (r = 0.04; p = 0.42), all subscales of sleep quality were significantly associated with fatigue (rrange = 0.20-0.59; all p values < 0.001).
Figure 1.
Schematic overview of bivariate Pearson correlations between chronotype, sleep quality, and cancer-related fatigue based on complete cases only. Abbreviation: PSQI = Pittsburgh Sleep Quality Index.
**p < 0.01. ***p < 0.001.
Table 3 shows the results of the hierarchical linear regression model after multiple imputation. Model 1 (R2 = 0.18; 95% confidence interval [CI] = 0.12-0.25) shows significant associations between fatigue after cancer and age (B = −0.06; p < 0.001), and comorbidities (B = 0.40; p < 0.001). These associations can be interpreted as follows: an increase of 10 years of age was associated with a decrease of 0.6 point in the VAS-fatigue scale; an increase of one comorbidity is associated with an increase of 0.40 points on the VAS-fatigue. No association was found between fatigue and intermediate aMS (B = 0.12; p = 0.67) or late aMS (B = −0.03; p = 0.90) compared with early aMS.
After inclusion of sleep quality variables, model 2 (R2 = 0.51, 95% CI = 0.44-0.57) shows significant associations between fatigue and subjective sleep quality (Bfairly good = 0.87; Bfairly bad = 1.47; Bvery bad = 2.80; for all p ≤ 0.001) and between fatigue and daily dysfunctioning (Bsome dysfunctioning = 2.15; Bquite a bit dysfunctioning = 3.09; Bsevere dysfunctioning = 3.28; all p values < 0.001). These associations can be interpreted as follows: the influence of subjective sleep quality ranged from an increase of 0.85 points (“fairly good sleep quality”) to 2.82 points (“very bad sleep quality”) on the VAS-fatigue relative to individuals who report their subjective sleep quality to be “very good”; the influence of daily dysfunctioning ranged from an increase of 2.03 points (“some dysfunctioning) to 3.28 points (“severe dysfunctioning”) on the VAS-fatigue compared with “no problems” in daily dysfunctioning. Compared with Model 1, the associations between fatigue and age and fatigue and comorbidities were no longer significant.
Potential multicollinearity issues of the sleep quality variables were evaluated by inspecting the variance inflation factor (VIF) and tolerance values of the second model applied on complete cases only. Two indicator (dummy) variables of the sleep disruption scale showed VIF (> 3.5) and tolerance (< 0.2) values that indicated a potential collinearity problem. However, these variables represent answer categories of the same categorical variable (sleep disruption) where the proportion of cases in the reference category is relatively small (5.4%), which causes the VIF to be larger. This was not the case for the other PSQI subscales where the proportion of cases in the reference categories were larger. The relatively large VIF of the dummy variables of the sleep disruption scale did not affect the other variables in the model and can therefore safely be ignored. There were no collinearity problems between subscales of the PSQI.
Similar results were obtained when the analysis was performed on the complete cases only (n = 379; see supplemental material Table S1). There was one difference: sleep duration answer “5-6 h” (Bimputed = −1.00, p = 0.01; Bcomplete cases = −1.41, p = 0.001) was significant in the complete cases analysis. Since confidence intervals were smaller for the imputed data analysis, these results were preferred.
Discussion
The aim of this study was to investigate the associations between chronotype and cancer-related fatigue and between sleep quality and cancer-related fatigue. Contrary to our hypothesis, the results do not support an association between chronotype and fatigue, measured by aMS. There were associations between two aspects of sleep quality and fatigue, specifically subjective sleep quality and daily dysfunctioning, indicating that a higher level of fatigue is associated with lower levels of self-reported sleep quality. Interestingly, we showed that fatigued survivors have comparable self-reported actual sleep times with those with no to mild fatigue but spend a longer time in bed trying to fall asleep. In addition, our results showed that survivors who are younger or have more comorbidities reported higher levels of fatigue after cancer. These associations attenuated when sleep quality was taken into account.
Previous studies on the association between chronotype and fatigue in other populations showed mixed results. One study showed that morning type individuals with irritable bowel symptoms reported less fatigue compared with evening types while this association was absent in healthy controls (Chrobak et al., 2018). Another study showed increased levels of chronic work-related fatigue in evening type student-workers compared with morning and intermediate types (Martin et al., 2012). However, in line with the current results, a recent study in patients with rheumatoid arthritis showed no association between chronotype and fatigue while these patients reported a 23 min earlier chronotype compared with the general population (Habers et al., 2020).
One explanation for these mixed results might be the use of different questionnaires to assess chronotype. The MCTQ assesses actual sleep times, but other questionnaires like the Morningness Eveningness Questionnaire (MEQ; Horne and Ostberg, 1975) and Composite Scale of Morningness (CSM; Smith et al., 1989) use preferred sleep times in ideal circumstances and statements to determine chronotype. The advantage of the MEQ and CSM is the cut-off score to determine chronotype. For the MCTQ, this determination is more arbitrary as there are no cut-off times to determine chronotype. To address the issue of mixed results, it is important to replicate the previous findings based on self-reported information with an objective assessment of circadian phase. Until now, this was difficult for large-scaled studies because the golden standard for this assessment is the assessment of Dim Light Melatonin Onset (DLMO). This procedure is very time-consuming. However, the BodyTime assay was introduced recently (Wittenbrink et al., 2018). This assay determines the circadian phase based on a single blood sample, which makes it more suitable for large-scaled studies.
Although the current results did not provide evidence for an association between chronotype and fatigue after cancer, our results do not contradict previous studies on circadian disruptions in cancer survivors (Mormont and Waterhouse, 2002; Rich, 2007; Payne, 2011; Innominato et al., 2014). The primary focus of the current study was to investigate whether the timing of actual sleep time, defined as chronotype, differed between survivors of cancer with and without fatigue. The studies on circadian disruptions looked more broadly at disruptions in rest-activity patterns (e.g., lying awake during the night and taking naps during the day to compensate) and showed that these disruptions were associated with cancer side effects like fatigue, sleep disturbances, depression, and cognitive impairment. Our results suggest a disturbed circadian rhythm in cancer patients with severe fatigue when we have a closer look to their sleep times. Results showed that survivors with moderate to severe fatigue tend to spend more time in bed before they fall asleep. One possible explanation is that fatigued survivors go to bed too early with respect to their circadian sleep drive. In other words, they might feel tired while their circadian rhythm is not yet set to sleep. Moreover, moderately and severely fatigued survivors reported more sleep disruptions compared with survivors without fatigue complaints.
Study Strengths and Limitations
As far as we know, the current study is the first to explore a potential association between circadian phase, defined as chronotype, and fatigue in cancer survivors. It is important to study this association as it provides more information on the optimal timing of light therapy as a treatment for fatigue after cancer. The results of this correlational study did not provide direct evidence that a delayed circadian phase is associated with fatigue after cancer. However, the results of the sleep times do not rule out that light therapy in the morning will improve fatigue in patients with a delayed circadian phase since moderate to severe fatigued survivors tend to take longer to get up in the morning and spend less time outside during the day. This suggests that they do not get early morning light, which is helpful to advance the circadian rhythm and will prepare them to fall asleep at an earlier time that might improve fatigue. Alternatively, light therapy might be able to improve fatigue by improving sleep quality, which was associated with cancer-related fatigue in our study. A recent study suggested an improvement of sleep quality after light therapy (Wams et al., 2017).
A second strength of this study was the use of aMS as an indicator for circadian phase instead of the original indicator of chronotype from the MCTQ. Originally, someone’s chronotype is based on the calculation of the midpoint between sleep onset and offset on free days when no alarm clock is used, corrected for sleep debt during the week, the midsleep on free days sleep corrected (MSFsc). However, recent results showed a stronger association between DLMO and aMS (Kantermann and Burgess, 2017) compared with the association between DLMO and MSFsc (Kantermann et al., 2015). For this reason, aMS was used.
There are also several limitations. First, this survey study was also used to recruit participants for a clinical trial to study the efficacy of light therapy as a treatment for fatigue after cancer. The possibility of participation in a trial to decrease fatigue could have been an additional reason to return a completed questionnaire for those suffering from fatigue. This might explain the high prevalence of 70% of fatigue in responders compared with 40% to 60% reported in literature (Oerlemans et al., 2013; Daniëls et al., 2014). Moreover, our sample included only survivors of (non-)HL, possibly reducing its generalizability to other populations of cancer survivors.
Second, all data were self-reported by the participants. Clinical variables (diagnosis, time since diagnosis, etc.) could not be verified. Also, we made a crude categorization of employment status based on the self-reported number of working days. We had no information on working hours. Consequently, survivors who were part-time employed could have been wrongly categorized as full-time employment. Moreover, previous studies showed that there is a discrepancy between subjective and objectively measured sleep information (Landry et al., 2015). Therefore, future studies should include objective measurements like the DLMO, BodyTime assay, or actigraphy to investigate the association between chronotype, sleep, and fatigue.
Third, the cross-sectional study design implies that our conclusions are based on associations. It is not possible to draw conclusions on the chronological order of the investigated variables in relation to cancer-related fatigue. This is relevant because it is likely that some variables are an effect of fatigue rather than a causal factor, for example, daily dysfunctioning. Longitudinal studies are necessary to provide more insight into the causality of associations found in the current study.
Future Research
Some of our findings give rise to interesting future research. First, the attenuated associations between fatigue and age and fatigue and comorbidities when sleep quality was added to the model suggest that sleep quality mediates these associations. The current study focused on the relationship between chronotype and cancer-related fatigue and sleep quality and cancer-related fatigue. Therefore, we did not perform mediation analyses to test this hypothesis. It is our recommendation that future research investigates a potential mediating effect of sleep quality while investigating factors that are associated with fatigue after cancer. Second, studies could investigate whether interventions aiming to improve sleep quality are more beneficial as a treatment for fatigue after cancer compared with interventions aiming to improve circadian phase.
Clinical Implications
This study suggests that fatigue after cancer is associated with subjective sleep quality and not with chronotype. For this reason, clinicians should not only focus on a patient’s timing of sleep and duration but also on the sleep quality reported by survivors of cancer. To do this, clinicians can ask questions like “How would you rate your sleep quality in the previous month: very good, fairly good, fairly bad, or very bad?” and “How often do you have trouble to stay awake while driving, eating meals, or engaging in social activities?” When a patient reports fairly bad or very bad sleep quality and problems to stay awake, further investigation of the sleep pattern and fatigue is necessary to determine the clinical significance of the fatigue. In case of clinical significant fatigue, patients should be referred for treatment (e.g., refer to cognitive-behavioral therapy [Gielissen et al., 2006] or receive sleep hygiene information).
Conclusion
The current study aimed to provide more insight into cancer-related fatigue by investigating associations between (1) fatigue and chronotype and (2) fatigue and sleep quality. As fatigue levels were related to sleep quality but not to chronotype, the results suggest that it is likely that fatigue is associated with disrupted sleep rather than circadian phase. More objectively measured circadian and sleep aspects are necessary to confirm this conclusion.
Supplemental Material
Supplemental material, sj-pdf-1-jbr-10.1177_0748730420987327 for Cancer-related Fatigue in Relation to Chronotype and Sleep Quality in (Non-)Hodgkin Lymphoma Survivors by Daniëlle E. J. Starreveld, G. Esther A. Habers, Heiddis B. Valdimarsdottir, Rob Kessels, Laurien A. Daniëls, Flora E. van Leeuwen and Eveline M. A. Bleiker in Journal of Biological Rhythms
Acknowledgments
We would like to thank Eefke Petersen (UMCU), Erik Marijt (LUMC), Lara Böhmer (HagaZiekenhuis), Eva de Jongh (ASZ), Cécile Janus (Erasmus MC), Margreet Houmes (ADRZ), and Marie José Kersten (Amsterdam UMC, location AMC) for the recruitment of participants. We would like to thank Marijke Gordijn (UMCG, Chrono@Work) and Eus van Someren (Amsterdam UMC and Netherlands Institute for Brain Research) for their advice on the MCTQ and PSQI. The Dutch Cancer Society (Grant Number NKI 2015-7909) financially supported this trial. The role of the Dutch Cancer Society is limited to peer review of the grant proposal. The Dutch Cancer Society is not involved in data collection, analyses, and interpretation of the data nor in the writing of the manuscript. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Conflict of Interest Statement: The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Daniëlle E. J. Starreveld  https://orcid.org/0000-0002-9381-7954
https://orcid.org/0000-0002-9381-7954
G. Esther A. Habers  https://orcid.org/0000-0002-0755-3076
https://orcid.org/0000-0002-0755-3076
Rob Kessels  https://orcid.org/0000-0002-2479-3872
https://orcid.org/0000-0002-2479-3872
Flora E. van Leeuwen  https://orcid.org/0000-0002-5871-1484
https://orcid.org/0000-0002-5871-1484
Supplemental material is available for this article online.
References
- Ancoli-Israel S, Liu L, Marler MR, Parker BA, Jones V, Sadler GR, Dimsdale J, Cohen-Zion M, Fiorentino L. (2006) Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer 14:201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Rissling M, Neikrug A, Trofimenko V, Natarajan L, Parker BA, Lawton S, Desan P, Liu L. (2012) Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support Care Cancer 20:1211-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KO, Getto CJ, Mendoza TR, Palmer SN, Wang XS, Reyes-Gibby CC, Cleeland C. (2003) Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage 25:307-318. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193-213. [DOI] [PubMed] [Google Scholar]
- Caruso D, Meyrel M, Krane-Gartiser K, Benard V, Benizri C, Brochard H, Geoffroy PA, Gross G, Maruani J, Prunas C, et al. (2020) Eveningness and poor sleep quality contribute to depressive residual symptoms and behavioral inhibition in patients with bipolar disorder. Chronobiol Int 37:101-110. [DOI] [PubMed] [Google Scholar]
- Chrobak AA, Nowakowski J, Zwolińska-Wcisło M, Cibor D, Przybylska-Feluś M, Ochyra K, Rzeźnik M, Dudek A, Arciszewska A, Siwek M. (2018) Associations between chronotype, sleep disturbances and seasonality with fatigue and inflammatory bowel disease symptoms. Chronobiol Int 35:1142-1152. [DOI] [PubMed] [Google Scholar]
- Coles T, Tan X, Bennett AV, Sanoff HK, Basch E, Jensen RE, Reeve BB. (2018) Sleep quality in individuals diagnosed with colorectal cancer: factors associated with sleep disturbance as patients transition off treatment. Psychooncology 27:1050-1056. [DOI] [PubMed] [Google Scholar]
- Daniëls L, Oerlemans S, Krol A, Creutzberg C, van de Poll-Franse L. (2014) Chronic fatigue in Hodgkin lymphoma survivors and associations with anxiety, depression and comorbidity. Br J Cancer 110:868-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortmann J, Fisher A, Hough R, Gregory A, Pugh G. (2018) Sleep quality, fatigue, and quality of life among teenage and young adult cancer survivors. J Adolesc Young Adult Oncol 7:465-471. [DOI] [PubMed] [Google Scholar]
- Gielissen MF, Verhagen S, Witjes F, Bleijenberg G. (2006) Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. J Clin Oncol 24:4882-4887. [DOI] [PubMed] [Google Scholar]
- Habers GEA, van der Helm AHM, Veldhuijzen DS, Allaart CF, Vreugdenhil E, Starreveld DEJ, Huizinga TWJ, Evers AWM. (2020) Earlier chronotype in patients with rheumatoid arthritis. Clin Rheumatol accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. (1975) A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4:97-110. [PubMed] [Google Scholar]
- Innominato PF, Roche VP, Palesh OG, Ulusakarya A, Spiegel D, Lévi FA. (2014) The circadian timing system in clinical oncology. Ann Med 46:191-207. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Garland SN, Carlson LE, Savard J, Simpson JSA, Ancoli-Israel S, Campbell TS. (2017) Bright light therapy improves cancer-related fatigue in cancer survivors: a randomized controlled trial. J Cancer Surviv 12:1-10. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Burgess HJ. (2017) Average mid-sleep time as a proxy for circadian phase. PsyCh J 6:290-291. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Sung H, Burgess HJ. (2015) Comparing the Morningness-Eveningness Questionnaire and Munich ChronoType Questionnaire to the dim light melatonin onset. J Biol Rhythms 30:449-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreissl S, Mueller H, Goergen H, Mayer A, Brillant C, Behringer K, Halbsguth TV, Hitz F, Soekler M, Shonukan O, et al. (2016) Cancer-related fatigue in patients with and survivors of Hodgkin’s lymphoma: a longitudinal study of the German Hodgkin Study Group. Lancet Oncol 17:1453-1462. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Best JR, Liu-Ambrose T. (2015) Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci 7:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, Hidalgo MPL, Allebrandt KV. (2011) Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int 28:771-778. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Cutler NL, Sack RL, Ahmed S, Thomas KH, Blood ML, Jackson JML. (1998) Morning vs evening light treatment of patients with winter depression. Arch Gen Psychiatry 55:890-896. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. (2006) The circadian basis of winter depression. Proc Natl Acad Sci U S A 103:7414-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JS, Hébert M, Ledoux É, Gaudreault M, Laberge L. (2012) Relationship of chronotype to sleep, light exposure, and work-related fatigue in student workers. Chronobiol Int 29:295-304. [DOI] [PubMed] [Google Scholar]
- Mock V, Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C, Donnelly J, Eisenberger M, Escalante C, Hinds P. (2000) NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park) 14:151-161. [PubMed] [Google Scholar]
- Mormont MC, Waterhouse J. (2002) Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol Int 19:313-323. [DOI] [PubMed] [Google Scholar]
- Mortimer JE, Barsevick AM, Bennett CL, Berger AM, Cleeland C, DeVader SR, Escalante C, Gilreath J, Hurria A, Mendoza TR. (2010) Studying cancer-related fatigue: report of the NCCN scientific research committee. J Natl Compr Canc Netw 8:1331-1339. [DOI] [PubMed] [Google Scholar]
- Oerlemans S, Mols F, Issa DE, Pruijt J, Peters WG, Lybeert M, Zijlstra W, Coebergh JWW, van de Poll-Franse LV. (2013) A high level of fatigue among long-term survivors of non-Hodgkin’s lymphoma: results from the longitudinal population-based PROFILES registry in the south of the Netherlands. Haematologica 98:479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Higgins C, Brady B, O’Connor B, Walsh D, Reilly R. (2018) The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer 26:3353-3364. [DOI] [PubMed] [Google Scholar]
- Oldenmenger WH, Pleun J, de Klerk C, van der Rijt CC. (2013) Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manage 45:1083-1093. [DOI] [PubMed] [Google Scholar]
- Payne JK. (2011) Altered circadian rhythms and cancer-related fatigue outcomes. Integr Cancer Ther 10:221-233. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013) R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. [Google Scholar]
- Redd WH, Valdimarsdottir H, Wu LM, Winkel G, Byrne EE, Beltre MA, Liebman ES, Erazo T, Hayes JA, Isola L. (2014) Systematic light exposure in the treatment of cancer-related fatigue: a preliminary study. Psychooncology 23:1431-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich TA. (2007) Symptom clusters in cancer patients and their relation to EGFR ligand modulation of the circadian axis. J Support Oncol 5:167-174. [PubMed] [Google Scholar]
- Roenneberg T, Pilz LK, Zerbini G, Winnebeck ECJB. (2019) Chronotype and social jetlag: a (self-) critical review. Biology 8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. (2003) Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 18:80-90. [DOI] [PubMed] [Google Scholar]
- Rubin DB. (1987) Multiple imputation for nonresponse in surveys. Hoboken (NJ): John Wiley. [Google Scholar]
- Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. (2003) The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Care Res (Hoboken) 49:156-163. [DOI] [PubMed] [Google Scholar]
- Schmidt ME, Semik J, Habermann N, Wiskemann J, Ulrich CM, Steindorf K. (2016) Cancer-related fatigue shows a stable association with diurnal cortisol dysregulation in breast cancer patients. Brain Behav Immun 52:98-105. [DOI] [PubMed] [Google Scholar]
- Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Penedo F, Lucci JA. (2013) Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun 30:S126-S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets E, Garssen B, Bonke B, De Haes J. (1995) The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 39:315-325. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. (1989) Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol 74:728-738. [DOI] [PubMed] [Google Scholar]
- Soreca I, Fagiolini A, Frank E, Goodpaster BH, Kupfer DJ. (2009) Chronotype and body composition in bipolar disorder. Chronobiol Int 26:780-788. [DOI] [PubMed] [Google Scholar]
- Starreveld DEJ, Daniels LA, Valdimarsdottir HB, Redd WH, de Geus JL, Ancoli-Israel S, Lutgendorf S, Korse CM, Kieffer JM, van Leeuwen F, et al. (2018) Light therapy as a treatment of cancer-related fatigue in (non-) Hodgkin lymphoma survivors (SPARKLE trial): study protocol of a multicenter randomized controlled trial. BMC Cancer 18:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K. (2011) Mice: multivariate imputation by chained equations in R. J Stat Softw 45:1-67. [Google Scholar]
- Wams EJ, Woelders T, Marring I, van Rosmalen L, Beersma DG, Gordijn MC, Hut RA. (2017) Linking light exposure and subsequent sleep: a field polysomnography study in humans. Sleep 40:zsx165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbrink N, Ananthasubramaniam B, Münch M, Koller B, Maier B, Weschke C, Bes F, de Zeeuw J, Nowozin C, Wahnschaffe A. (2018) High-accuracy determination of internal circadian time from a single blood sample. J Clin Invest 128:3826-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jbr-10.1177_0748730420987327 for Cancer-related Fatigue in Relation to Chronotype and Sleep Quality in (Non-)Hodgkin Lymphoma Survivors by Daniëlle E. J. Starreveld, G. Esther A. Habers, Heiddis B. Valdimarsdottir, Rob Kessels, Laurien A. Daniëls, Flora E. van Leeuwen and Eveline M. A. Bleiker in Journal of Biological Rhythms