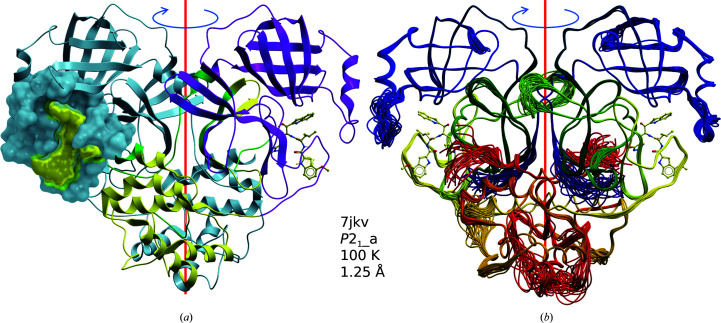
Figure 1.
High-resolution structure and dynamics of SARS-CoV-2 3CLpro, exemplified by PDB entry 7jkv (1.25 Å resolution) from the P21_a polymorph (Table 2 ▸). (a) Cartoon model of the dimer with the vertical twofold NCS axis (red line) in the plane of the paper. In the left protomer (light blue), the binding-pocket surface (blue surface) around the covalently bound inhibitor GRL2420 (yellow surface) is highlighted. In the right protomer, the catalytic domain is highlighted in purple, residues involved in dimer contacts are colored green and the remaining regions are in yellow. (b) Visualization of protein plasticity through an ensemble of 25 molecular-dynamics traces obtained from multi-conformer refinement with Phenix (Burnley et al., 2012 ▸). The backbone ‘worms’ of the models are colored from the N-terminus (blue) to the C-terminus (red). The relative rigidity of the binding pocket is clearly visible compared with regions of increased anisotropic movement such as some loops in the catalytic domain, parts of the C-terminal regions (orange to red) and the N- and C-termini. N-terminal tags are common and are distant from crystal contacts. In contrast, in the single structure with a C-terminal His6 tag (PDB entry 6wtt, space group P3221), only the C-terminus is exposed and disordered, while the N-terminal Ser1 participates in dimer contacts and in close crystal contacts, leaving no room for an N-terminal tag. This figure was generated with ICMPro (Abagyan et al., 1994 ▸).