Figure 1.
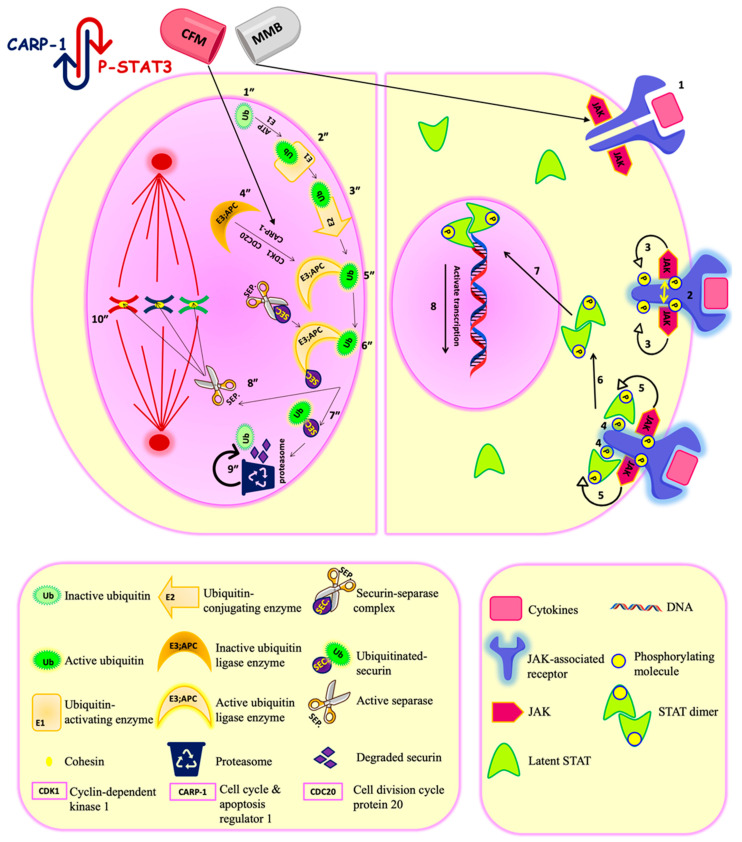
Momelotinib and CFM4.16 synergistic combination. Momelotinib is a JAK-STAT inhibitor. JAK-STAT pathway is a direct signaling pathway transfer the signal from extracellular to nucleus to control the expression of certain genes. The upregulation of the JAK-STAT pathway is the key player in different cancers, and its regulation is under clinical investigation for cancer therapy. The principal components of this pathway are: cytokines-receptor complex, JAK, and STAT proteins. Mechanistically, when the ligand bind to its corresponding JAK-associated receptor (1), the receptors arms are brought into proximity, which enables transphosphorylation between the two JAK molecules (2). The activated phosphorylated JAK subsequently phosphorylates the receptor arms, which is the binding site for the latent transcription factors STAT (3). After the STAT molecules bind to the receptor arms (4), they become ready for phosphorylation by JAK (5). Once phosphorylated, the two STAT monomers dimerize through reciprocal phosphotyrosine-SH2 domain interaction (6). The STAT dimer is an active transcriptional factor that is translocated to the nucleus (7) and binds to a specific DNA sequence in the target gene promoters by DNA-binding domain to control transcription of specific genes (8). Momelotinib antagonizes the ATP binding to JAK1/2, leading to inhibition of the JAK-STAT pathway. CFM4.16 is CARP-1/APC/C interaction inhibitor. APC/C is E3 ubiquitin ligase responsible for tagging cell cycle proteins for proteasomal degradation for the metaphase/ anaphase cell cycle transition. Aberrant APC/C system is associated with cancer progression. Mechanistically, the process starts with latent ubiquitin (Ub) molecules present in the cells (1″), which is activated by Ub-activating enzymes (E1) in an ATP-dependent manner(2″). The activated (Ub)will be transferred to a Ub-conjugating enzyme (E2) (3″), which will conjugate the Ub molecules to activated Ub ligase (E3)/APC/C. The E3/APC/C is under the control of CDK-1(cyclin-dependent kinase-1), CARP-1, and CDC 20 (cell division cycle protein 20).The CDK-1 phosphorylates the APC/C, while CARP-1 binds to the APC2 subunit of APC/C for coactivation. Then the phosphorylated APC/C will bind to CDC 20 to be fully activated (4″). Then The (E2) conjugates the Ub molecules to activated Ub ligase (E3)/(APC/C) (5″). Activated APC/C system ubiquitinates the securin protein (chaperone) to be marked for proteasomal degradation(6″&7″). After the degradation of securin, the separase (separin) will be activated and will break down the cohesin protein between two sister chromatids (8″). Break down of cohesin will lead to the separation of two sister chromatids in anaphase (10″). Thus, APC/C is responsible for maintaining normal chromosome number and genetic stability. Also, APC/C is responsible for turning over S/M cyclins to terminate mitosis. The proteasome catalytic unit will degrade the tagged protein into a small peptide chain and Ub. The Ub will be reused, and the fate of the peptide chain will depend on the cell needs; either it will be repurposed for protein synthesis or energy production (9″). The simultaneous down-regulation of STAT3 and APC/C activation is the underlying mechanism for their synergism.