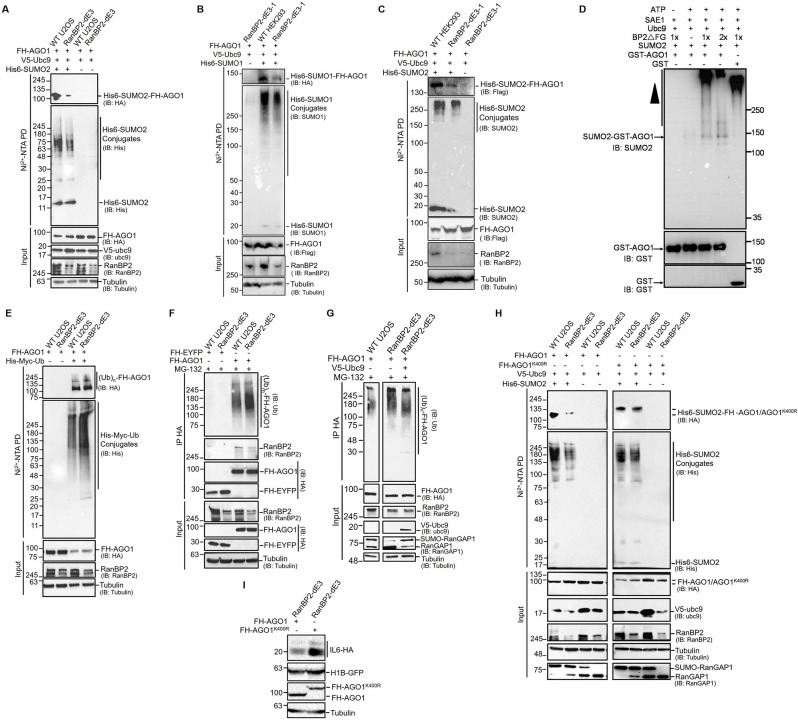
Fig 7. RanBP2 promotes the sumoylation and inhibits the ubiquitination of AGO1.
(A) Unmodified and RanBP2-dE3 U2OS cells were co-transfected with plasmids expressing Flag-HA-tagged AGO1 (FH-AGO1), SV5-tagged Ubc9 (V5-Ubc9), and His-tagged SUMO2 (His6-SUMO2 “+”) or control vector (His6-SUMO2 “-”). 24 h post-transfection cells were lysed in 6 M Guanidinium-HCl, and the His6-SUMO2 conjugates were isolated on Nickel beads (“Ni2+ NTA PD”) or the lysates were directly analyzed (“input”) and separated by SDS-PAGE. Conjugates were analyzed for the presence of FH-AGO1 by immunoblotting for HA (IB: HA), and for total His6-SUMO2 conjugates by immunoblotting for His (IB: His). Input lysates were immunoblotted with antibodies against FH-AGO1, V5-Ubc9, RanBP2 and α-tubulin. See S10A Fig for the quantification of His6-SUMO2-FH-AGO1 levels. (B-C) As in (A), except that unmodified and RanBP2-dE3-1 HEK293 cells were transfected with His6-SUMO1 (B) or His6-SUMO2 (C). Antibodies used for immunoblotting were as indicated on the right. See S10B and S10C Fig for the quantification of His6-SUMO1-FH-AGO1 and His6-SUMO2-FH-AGO1 levels. (D) AGO1 was in vitro sumoylated with purified components, with SUMO2, active recombinant human RanBP2 protein (BP2ΔFG) as the SUMO E3-ligase, and recombinant GST-tagged human AGO1 protein (GST-AGO1) or GST as substrates. Equal amounts of SAE1, SUMO2, Ubc9, GST-AGO1 and GST (35 ng) were added to 10 μL reactions, where 1x is estimated to be 5 ng of BP2ΔFG. Negative controls lacking ATP or BP2ΔFG were also shown. After incubation at 30°C for 1 h, the reactions were analyzed by immunoblotting with antibodies indicated. The position of SUMO2-modified AGO1 is indicated and the SUMO2-conjugated BP2ΔFG is represented by the arrowhead. (E) Similar to (A), except that U2OS cells were co-transfected with plasmids for FH-AGO1, and His-Myc-tagged ubiquitin (His-Myc-Ub “+”) or control vector (His-Myc-Ub “-”). 18 h post-transfection, cells were treated with MG132 (10 μM) for an additional 7 h to preserve ubiquitinated conjugates. Cells were lysed in 6 M Guanidinium-HCl, and the His-Myc-Ub conjugates were isolated on Nickel beads (“Ni2+ NTA PD”) or the lysates were directly analyzed (“input”) and separated by SDS-PAGE. Conjugates were analyzed for the presence of FH-AGO1 by immunoblotting for HA (IB: HA), and for total His-Myc-Ub conjugates by immunoblotting for His (IB: His). See S10D Fig for the quantification of His-Myc-Ub-FH-AGO1 levels. (F) Unmodified and RanBP2-dE3 U2OS cells were transfected with plasmids for FH-AGO1 or Flag-HA-tagged yellow fluorescent protein (FH-EYFP). 18 h post-transfection, cells were treated with MG132 (10 μM) for an additional 7 h to preserve ubiquitinated conjugates. Cells were lysed in RIPA buffer, and FH-AGO1/FH-EYFP and associated proteins were isolated by immunoprecipitation using anti-HA antibodies (“IP HA”) or the lysates were directly analyzed (“input”) and separated by SDS-PAGE. The immuoprecipitates were analyzed by immunoblotting for ubiquitinated proteins (IB: Ub), immunoprecipitated FH-AGO1/FH-EYFP by immunoblotting against HA, and for co-immunoprecipitated RanBP2. Input lysates were immunoblotted for RanBP2, FH-AGO1 and FH-EYFP (IB: HA) and α-tubulin. See S10E Fig for the quantification of (Ub)n-FH-AGO1 levels. (G) As in (F), except that unmodified and RanBP2-dE3 U2OS cells were co-transfected with FH-AGO1 and either V5-Ubc9 to enhance sumoylation or control plasmid. The immuoprecipitates were analyzed for ubiquitinated proteins by immunoblotting against ubiquitin (IB: Ub). Input lysates were immunoblotted for FH-AGO1 (IB: HA), RanBP2, V5-Ubc9, RanGAP1 and α-tubulin. Note that Ubc9 overexpression rescued RanGAP1-sumoylation in RanBP2-dE3 cells and decreased the amount of ubiquitinated FH-AGO1. See S10F Fig for the quantification of (Ub)n-FH-AGO1 levels. (H) As in (A), except that cells were transfected with either FH-AGO1 or FH-AGO1K400R. Note that FH-AGO1K400R was no longer sumoylated in a RanBP2-dependent manner. (I) RanBP2-dE3 U2OS cells were co-transfected with plasmids expressing an intron-containing IL6-HA construct (IL6-1i-HA), histone 1B-GFP (H1B-GFP) and either FH-AGO1 or FH-AGO1K400R. Cell lysates were collected 24 h post-transfection, separated by SDS-PAGE and immunoblotted with antibodies against HA (IL6-HA, FH-AGO1 and FH-AGO1K400R), GFP and α-tubulin. See S11A Fig for the quantification of IL6-HA levels.