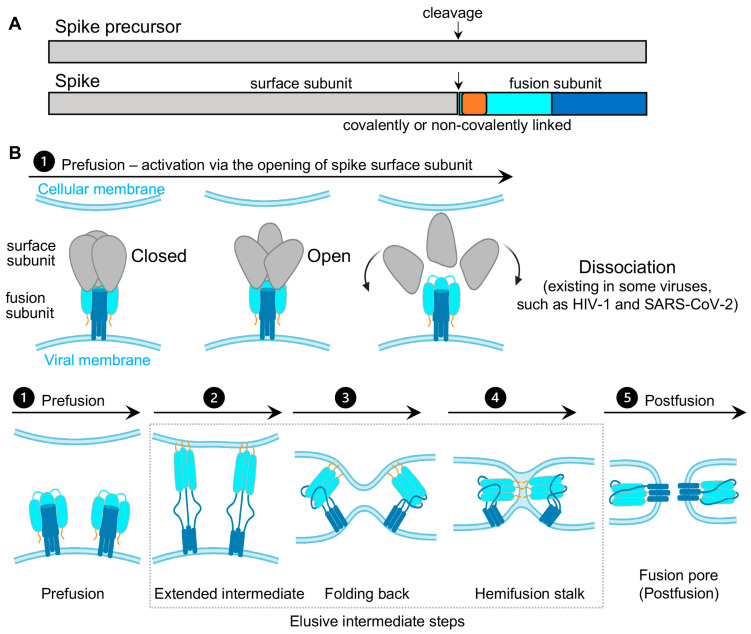
Figure 1.
Class I viral fusion proteins and proposed model of viral membrane fusion. (A) Schematic drawing of spike precursor and cleaved spike. The spike protein is initially synthesized as a single-chain polypeptide (spike precursor) and later cleaved into a trimer of covalently or non-covalently linked heterodimer. The heterodimer consists of the surface receptor-binding subunit (gray) and the fusion subunit (fusion peptide or fusion loop (FP/FL), dark yellow; N-terminal domain, cyan; C-terminal domain, dark blue). (B) Proposed conformational events of virus spikes during viral membrane fusion. These events are as follows, involving conformational changes in the surface subunit (top row) and changes in the fusion subunit (low row, simplified by only showing the fusion subunit [1]). (1) Prefusion—conformations of the spike in “closed” and open forms. Spike activation proceeds through an opening of the trimer, usually in response to binding to receptor or due to a cellular cue such as low pH. For non-covalently linked spikes, dissociating/decoupling between the surface/exterior subunit with the fusion subunit has been observed/suggested after the spike opens, such as HIV-1 and SARS-CoV-2 spikes. FP/FL remains sequestered in this process. (2) Exposing, extending, and inserting the FP/FL into the cellular membrane leads to the formation of an extended prehairpin intermediate. (3) Folding back the C-terminal segment of the fusion subunit back on the N-terminal segment core brings viral and cellular membranes into proximity. (4) Further folding and dragging two membranes into contact promotes two membranes’ merging to form a hemifusion stalk. (5) The fusion subunit folds into a stable post-fusion conformation, allowing a fusion pore to form. The intermediate steps from (2) to (4) remain elusive. This proposed model does not specify or speculate the number of spikes required for fusion pore formation.