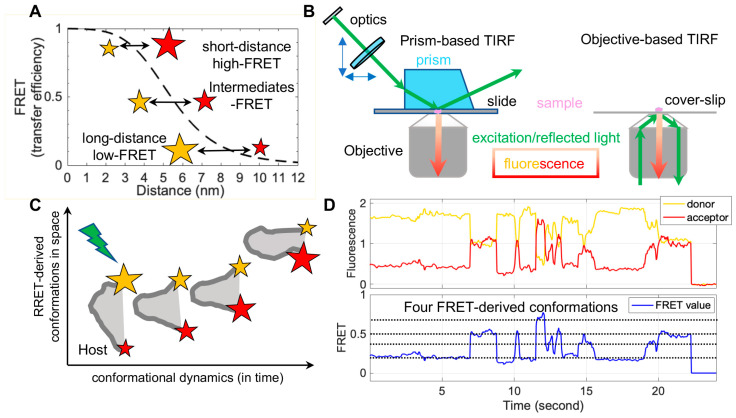
Figure 2.
Single-molecule Förster resonance energy transfer (smFRET) principle, instrumentation, and imaging of dynamic biomolecules. (A) An example curve depicting energy transfer efficiency (Förster resonance energy transfer (FRET), dashed black line) from an excited donor fluorophore to a neighboring acceptor fluorophore as a function of donor–acceptor distances. FRET values or FRET negatively correlate with the distance within a couple of nanometers between a donor (yellow star) and an acceptor (red star). (B) Widely used smFRET imaging instrumentations: prism- and objective-based total internal reflection fluorescence (TIRF). (C,D) Real-time observations of conformational motions in biomolecules by smFRET. (C) Diagram depicting ideal FRET-derived space-time coordinates of biomolecule conformations. Donor, yellow star; acceptor, red star; relative fluorescence intensity, the star’s size; biomolecule of interest, gray. (D) Example FRET-related traces showing four interconvertible conformations in real time. The host molecule dynamically samples four conformations, reflected by different donor–acceptor energy transfer efficiencies. Donor fluorescence trace, solid yellow line; acceptor fluorescence trace, solid red line; calculated FRET trace, solid blue line; FRET-indicated conformations, dashed black lines.