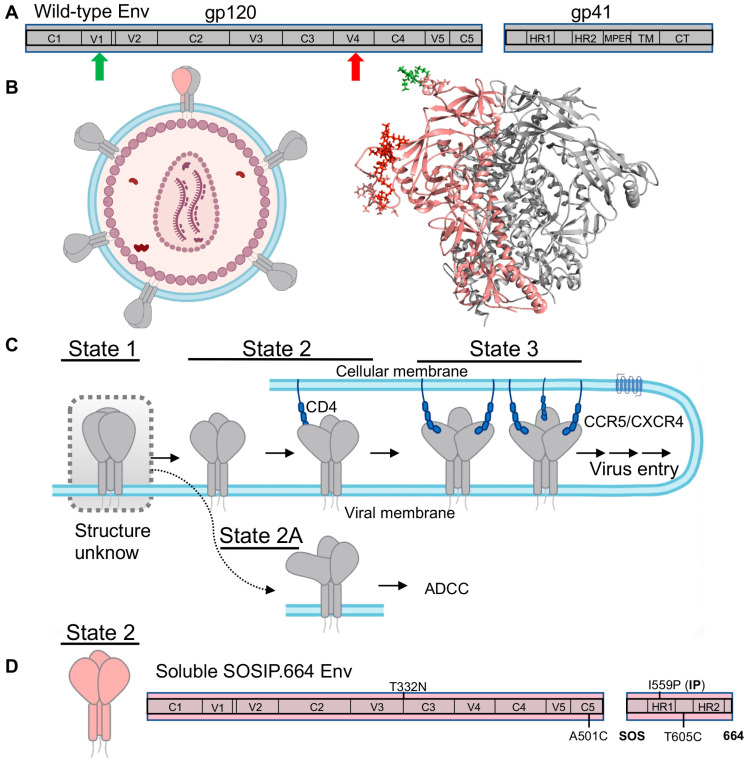
Figure 4.
On-path pre-fusion HIV-1 envelope (Env) trimer conformations identified by smFRET imaging. (A) Scheme of wild-type HIV-1 Env, noncovalently associated gp120 and gp41 subunits. (B) smFRET imaged individual HIV-1 viruses carrying a fluorescently labeled protomer within an Env trimer and elsewhere wild-type trimers. The Cy3/Cy5-labeled protomer (Cy3, green; Cy5, red) is in pink, whereas other wild-type protomers are colored gray. Structure is made based on PDB accessions 4ZMJ and 5FUU. (C) Model of Env activation by sequential binding of CD4 receptors. Env dynamically samples three primary conformational states in which State 1 is the predominant one. Upon sequential activation by CD4, Env transits through an asymmetric State 2 to a completely open State 3 (two- or three-CD4-bound trimer). State 2-Env is a single CD4-bound asymmetric trimer, in which the CD4-bound protomer adopts State 3 and the neighboring free protomers adopt State 2. State 2A is an off-path conformation that is highly vulnerable to antibody-dependent cellular cytotoxicity (ADCC). Following CD4 activation, the binding of coreceptors CCR5/CXCR4 lead to virus entry, which smFRET has not informed. (D) Vaccine candidates based upon soluble SOSIP.664 Env trimer resemble State 2, consisting of three State 2 protomers. The design of soluble SOSIP.664 Env is illustrated in the schematic. Results from (D) infer that State 1 Env (C) is structurally unknown.