Figure 1. Schematic of the self-blinding microdosing experiment.
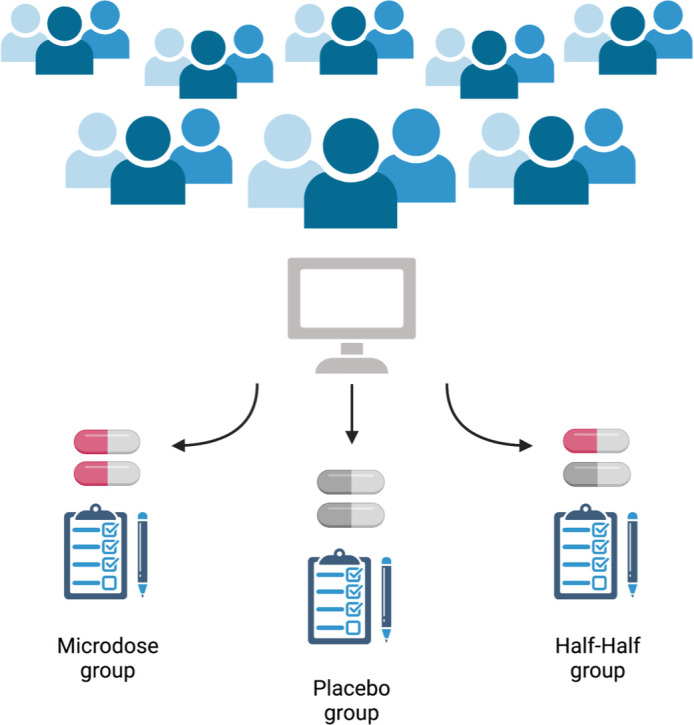
Participants signed up to the study on https://selfblinding-microdose.org/ (top), and were provided with instructions on how to perform the experiments. Szigeti et al. sent the participants opaque capsules, zip bags, envelopes and QR codes. First, the participants prepared microdoses by placing drugs into the opaque capsules. Empty capsules were used as placebos. Once the microdoses and placebos were prepared, sets of capsules for each week of the trial were assembled according to the dose schedule, and each capsule was placed in a zip bag with a label indicating what day of the week it should be taken. Every participant prepared eight sets of weekly capsules, four ‘microdose’ sets and four ‘placebo’ sets (participants had to be unaware of whether they were taking the placebo, the drug, or a half-dose, so it was important that they prepare for any of the three regimes). Each weekly set of capsules was then placed into an envelope along with a single QR code that identified whether the envelope contained placebo or microdose capsules (the QR code was used by Szigeti et al. afterwards to determine what each participant had taken). These envelopes were then placed in pairs into four big envelopes. Each big envelope contained either two microdose sets of capsules or two placebo sets of capsules. The big envelopes were then shuffled and two were chosen using a semi-randomized drawing process. The other two big envelopes were discarded. The drawing process was designed so that each participant would have a one in three chance of drawing envelopes matching one of the three possible regimes (bottom): either a full microdose regime (both big envelopes contained microdose weekly sets), a half microdose regime (one big envelope contained microdose weekly sets and one contained placebo weekly sets), or a placebo regime (both big envelopes contained placebo weekly sets). This resulted in three experimental groups of approximately the same number of participants following each regime, without the participants themselves knowing what they were taking. This approach to setting up the study allowed participants to blind themselves to what they were taking, while at the same time overcoming the financial and regulatory hurdles associated with drug studies.
