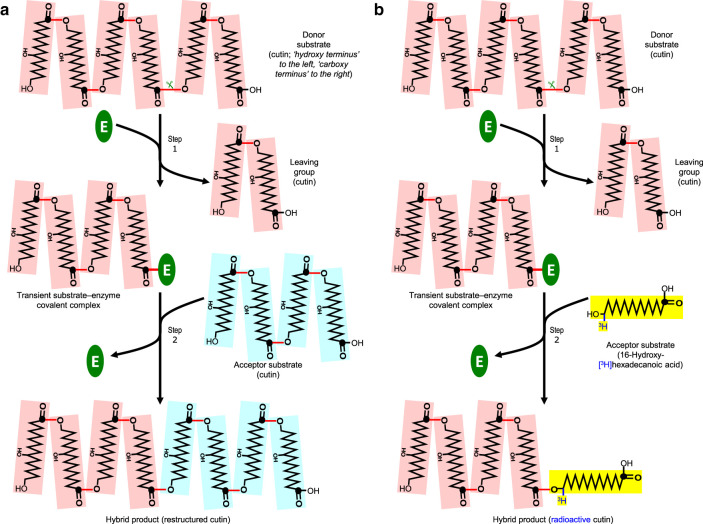
Figure 1. The proposed cutin:cutin transacylation mechanism.
(a) The reaction as proposed to occur naturally in vivo. In step 1, a cutin chain (donor substrate, pink) is cut ( ) by a transacylase (E), forming a transient cutin–enzyme covalent complex and releasing the carboxy-terminal portion of the cutin as a leaving group. In step 2, after incremental rearrangement of the cuticular network, the enzyme-bonded portion of cutin is transferred onto a free –OH group of a neighbouring cutin molecule (acceptor substrate, pale blue), restoring the strength of the cutin and releasing the enzyme for the next cycle. (b) As (a) but showing the reaction occurring in situ when an exogenous soluble radiolabelled acceptor substrate (16-hydroxy-[3H]hexadecanoic acid, shown in yellow) is infiltrated into epidermal walls containing endogenous enzyme plus endogenous insoluble cutin. An insoluble radiolabelled ‘hybrid’ product is formed, which is assayed by scintillation counting. Key: ⬤, carbon-1 of a hydroxy-fatty acid moiety; red line (—), ester bond; (E), transacylase. For simplicity, the cutin molecules depicted are small, unbranched and composed entirely of 10,16-dihydroxyhexadecanoic acid residues; however, natural cutin is likely to be branched via the 10-hydroxy groups and to be of higher degree of polymerisation than shown. The cutin chains are drawn with the ‘hydroxy-terminus’ to the left and the ‘carboxy-terminus’ to the right.
) by a transacylase (E), forming a transient cutin–enzyme covalent complex and releasing the carboxy-terminal portion of the cutin as a leaving group. In step 2, after incremental rearrangement of the cuticular network, the enzyme-bonded portion of cutin is transferred onto a free –OH group of a neighbouring cutin molecule (acceptor substrate, pale blue), restoring the strength of the cutin and releasing the enzyme for the next cycle. (b) As (a) but showing the reaction occurring in situ when an exogenous soluble radiolabelled acceptor substrate (16-hydroxy-[3H]hexadecanoic acid, shown in yellow) is infiltrated into epidermal walls containing endogenous enzyme plus endogenous insoluble cutin. An insoluble radiolabelled ‘hybrid’ product is formed, which is assayed by scintillation counting. Key: ⬤, carbon-1 of a hydroxy-fatty acid moiety; red line (—), ester bond; (E), transacylase. For simplicity, the cutin molecules depicted are small, unbranched and composed entirely of 10,16-dihydroxyhexadecanoic acid residues; however, natural cutin is likely to be branched via the 10-hydroxy groups and to be of higher degree of polymerisation than shown. The cutin chains are drawn with the ‘hydroxy-terminus’ to the left and the ‘carboxy-terminus’ to the right.