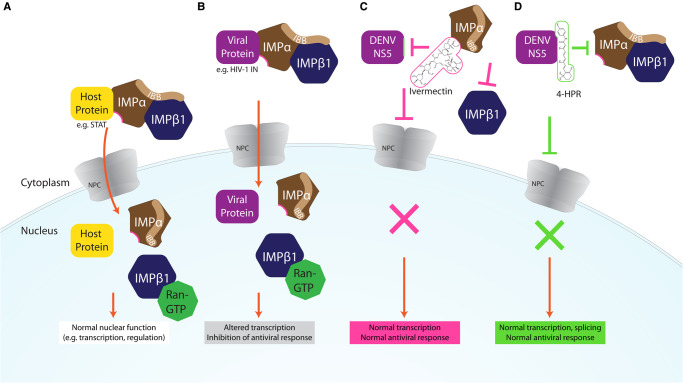
Figure 1. Schematic representation of the host IMPα/β1-dependent nuclear import pathway (A), showing how it is co-opted by viral proteins in viral infection (B), and how small molecule antivirals can impact the pathway (C,D).
(A) Host proteins (e.g. transcription factor STAT-2) contain nuclear localisation signals which are recognised by IMPα (brown), after the autoinhibitory IMPβ-binding (IBB) domain of IMPα binds IMPβ1, forming the IMPα/β heterodimer. This complex is then translocated across the nuclear pore complex (NPC), and the cargo is released after the binding of Ran-GTP to IMPβ1 dissociates the complex. The cargo can then carry out its normal nuclear function, such as transcriptional regulation of the antiviral response. (B) During viral infection, specific NLS-containing viral proteins (e.g. HIV-1 IN, purple) are imported into the nucleus by the same IMPα/β1 dependent mechanism, where they can interfere with normal cellular functions, such as altering transcription to antagonise the antiviral response in order to maximise the rate of virus production. (C) Ivermectin (pink) binds IMPα, dissociating the IMPα/β1 heterodimer and preventing binding to its viral (as well as host) protein target(s), thereby preventing its nuclear import and downstream transcriptional effects. (D) 4-HPR (green) specifically binds viral protein DENV2 NS5, preventing binding of the IMPα/β1 and nuclear import, and associated downstream effects on transcription and splicing.