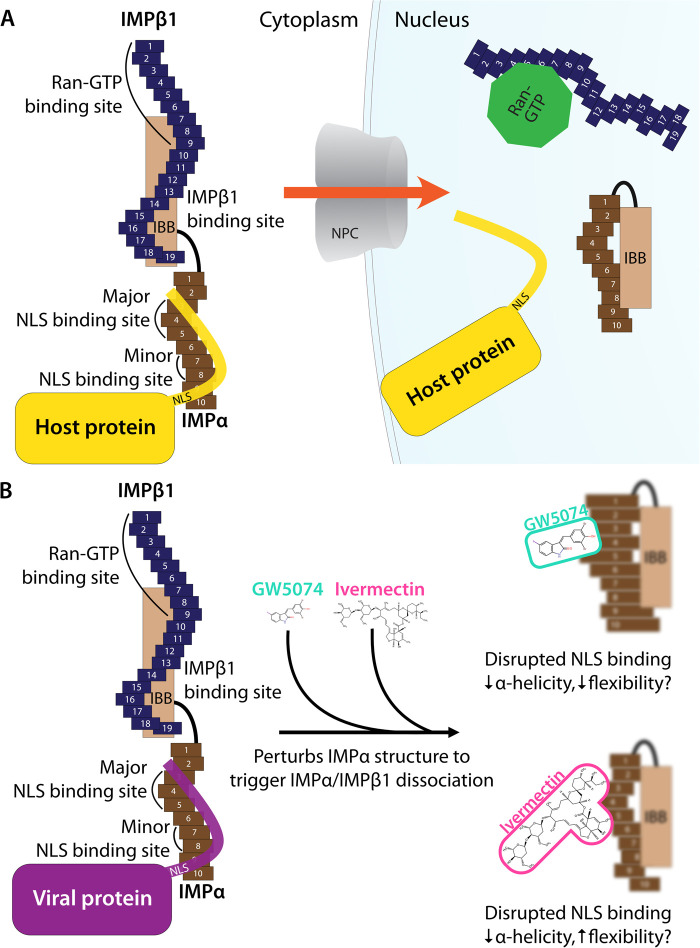
Figure 2. Schematic representation of IMPα and IMPβ1 structural/functional domains; impact of inhibitors.
(A) Schematic representation of the IMPα/β1 complex based on the crystal structures of human IMPα and IMPβ1. IMPα comprises 10 armadillo (ARM) repeats and an IMPβ-binding (IBB) domain. IMPα ARM repeats 2–4 form the major cargo NLS binding site, while ARM 7–8 form the minor NLS binding site. IMPβ1 comprises 19 HEAT repeats, where repeats 7–19 bind the IMPα IBB domain, and Ran-GTP binds to the N-terminus/repeat 8. In the cytoplasm, IMPβ1 (blue) binds IMPα (brown) via the IBB domain, exposing the major NLS binding site for cargo protein (yellow) binding. After transport into the nucleus across the NPC, Ran-GTP binds to IMPβ1, altering its conformation and displacing and releasing the IBB domain, which then binds the NLS binding sites in an autoinhibitory manner, preventing NLS binding. (B) Ivermectin and GW5074 bind directly to IMPα to perturb its structural integrity as confirmed by biophysical measurements including circular dichroism, thermostability analysis, and analytical ultracentrifugation [44,47], to reduce IMPα-helicity/impact flexibility, and thereby prevent IMPα binding to NLS-containing proteins or IMPβ1.