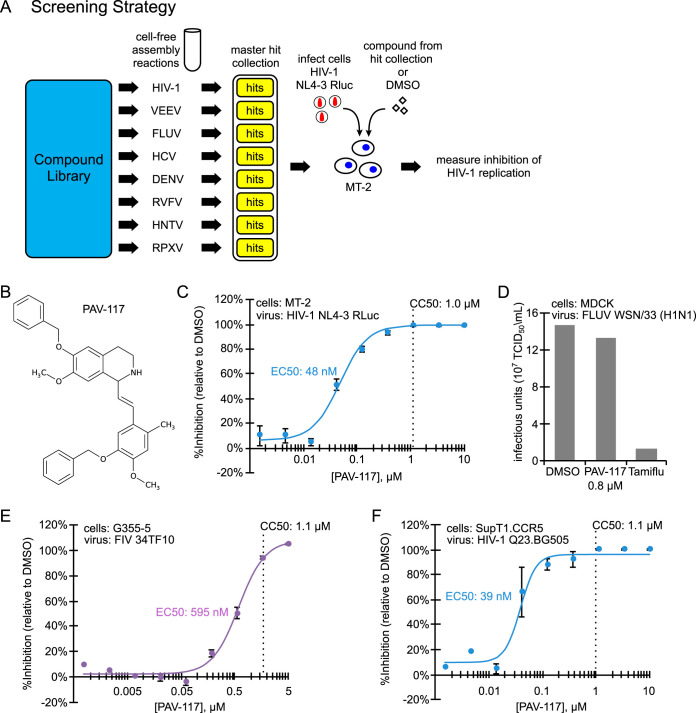
FIG 2.
PAV-117, identified using cell-extract-based assembly screens, inhibits replication of HIV-1, but not FIV or FLUV, in cell culture. (A) Schematic showing the cell-free assembly screening strategy used to identify PAV-117. Small molecules from a compound library of 150,000 compounds were assayed in cell-free screens that recapitulate assembly of eight different viral capsids, including HIV-1, Venezuelan equine encephalitis virus (VEEV), influenza A virus (FLUV), hepatitis C virus (HCV), dengue virus (DENV), Rift Valley fever virus (RVFV), Hantaan virus (HNTV), and rabbit pox virus (RPXV). Each screen is analogous to the HIV-1 screen (see Fig. 1C). Results from the library screens led to generation of a master hit collection of 249 small molecules that displayed inhibitory activity in one or more of the eight cell-free assembly screens. Compounds in the master hit collection were assayed for inhibition of HIV-1 replication in MT-2 T cells, with PAV-117 identified as the compound with optimal EC50, CC50, and other characteristics in the MT-2 assay. (B) Chemical structure of PAV-117, a tetrahydroisoquinolone. (C) To determine the EC50 of PAV-117 against HIV-1 replication, a dose-response curve for inhibition of HIV-1 replication by PAV-117 was generated by treating human MT-2 T cells with the indicated doses of PAV-117, followed by infection with a replication-competent HIV-1 NL4-3 RLuc reporter virus (MOI of 0.02). After 96 h of spreading infection, luciferase activity was measured as an indicator of HIV-1 replication and is displayed as the inhibition of replication relative to DMSO-treated controls (% inhibition). The CC50 was determined in parallel using uninfected MT-2 T cells and is marked by a vertical dashed line. Error bars show the SEM determined from three replicates. (D) Graph showing quantification of infectious FLUV in MDCK cells treated with DMSO or with either PAV-117 or Tamiflu at a concentration 20-fold higher than the EC50 for each drug (0.8 μM for PAV-117, 10 μM for Tamiflu). Treated cells were infected with FLUV (strain WSN/33 [H1N1], MOI of 0.001), and the viral titers were measured after 24 h by TCID50 and are shown as infectious units (107 TCID50 U/ml). (E) To determine the EC50 of PAV-117 against FIV replication, a dose-response curve for the inhibition of FIV replication by PAV-117 was generated by treatment of feline G355-5 cells with the indicated doses of PAV-117, followed by infection with FIV 34TF10 (1,954 nU of RT activity per well). After 144 h of spreading infection, RT activity was measured and used to calculate inhibition of FIV replication relative to DMSO controls (% inhibition). The CC50 was determined in parallel using uninfected G355-5 cells and is marked by a vertical dashed line. Error bars show the SEM determined from three replicates. (F) Quantification of replication of an HIV-1 primary isolate. To determine the EC50 of PAV-117 against HIV-1 replication, a dose-response curve for inhibition of HIV-1 replication by PAV-117 was generated by treating human SupT1.CCR5 cells with the indicated doses of PAV-117 or DMSO, followed by infection with HIV-1 Q23.BG505, a CCR5-tropic subtype A molecular clone. HIV-1 replication proceeded for 96 h and was followed by measurement of HIV-1 infectivity in the culture supernatant using the MUG assay in TZM-bl cells.