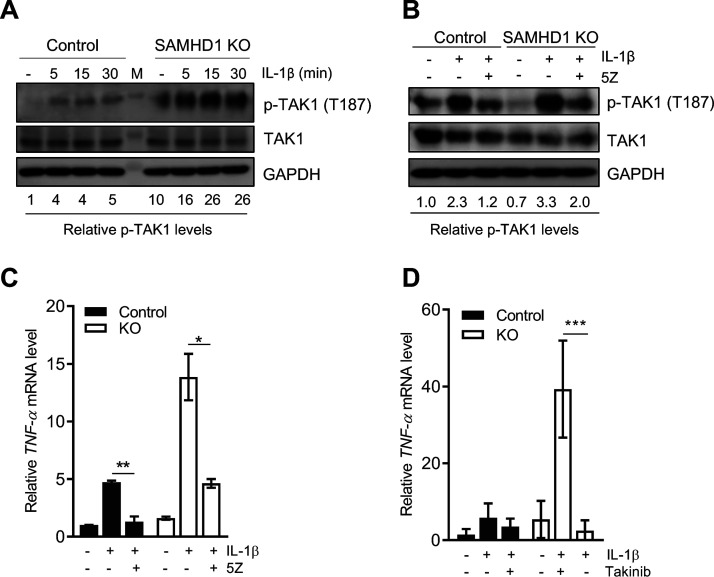
FIG 1.
Endogenous SAMHD1 inhibits TAK1 activation. (A) THP-1 control and SAMHD1 KO cells were seeded in low-glucose (5.5 mM) media for 48 h. Cells were mock treated or treated with IL-1β, harvested at 5, 15, and 30 min, and then lysed for immunoblotting. GAPDH was used as a loading control. Relative p-TAK1 proteins levels were calculated by densitometry analysis with normalization to total TAK1 levels and GAPDH. Mock-treated cells were set as 1. (B and C) THP-1 control and KO cells were grown as in panel A. Cells were then cultured in 5Z (1 μM) or DMSO for 30 min. After 5Z removal, IL-1β was added to cells for 5 min prior to collection and analysis by immunoblotting (B) or for 2 h for RT-qPCR detection of TNF-α mRNA (C). Relative p-TAK1 protein levels were calculated by densitometry analysis. The p-TAK1 signal was normalized to total TAK1 protein and GAPDH. Untreated control cells (without inhibitor or IL-1β stimulation) were set as 1. (A and B) The immunoblots were representative data of three independent experiments. (C) Measurement of mRNA levels was performed from samples in the same experiment described in panel B. TNF-α mRNA was normalized to spliced GAPDH. Data represent duplicate samples, and error bars depict standard deviation (SD). Statistical significance was calculated by unpaired t test. (D) Cells were treated with Takinib (10 μM) for 2 h prior to IL-1β stimulation. TNF-α mRNA was quantified by RT-qPCR as in panel C. Data represents triplicate samples, and error bars depict standard error of the mean (SEM). Statistical significance was calculated by unpaired t test. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.