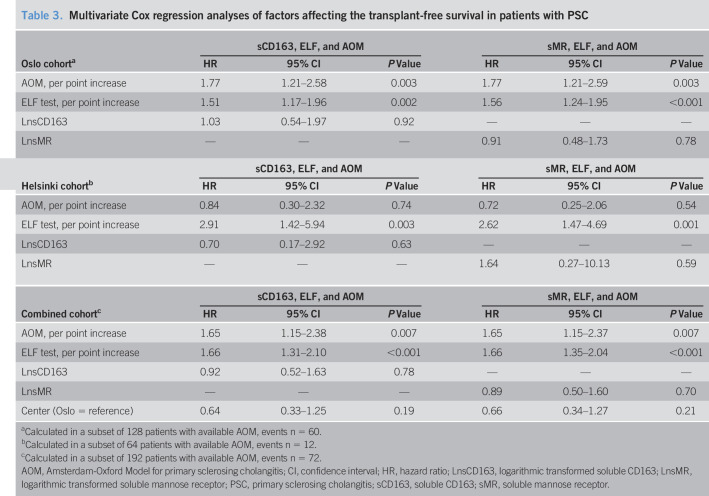
Table 3.
Multivariate Cox regression analyses of factors affecting the transplant-free survival in patients with PSC
| Oslo cohorta | sCD163, ELF, and AOM | sMR, ELF, and AOM | ||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| AOM, per point increase | 1.77 | 1.21–2.58 | 0.003 | 1.77 | 1.21–2.59 | 0.003 |
| ELF test, per point increase | 1.51 | 1.17–1.96 | 0.002 | 1.56 | 1.24–1.95 | <0.001 |
| LnsCD163 | 1.03 | 0.54–1.97 | 0.92 | — | — | — |
| LnsMR | — | — | — | 0.91 | 0.48–1.73 | 0.78 |
| Helsinki cohortb | sCD163, ELF, and AOM | sMR, ELF, and AOM | ||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| AOM, per point increase | 0.84 | 0.30–2.32 | 0.74 | 0.72 | 0.25–2.06 | 0.54 |
| ELF test, per point increase | 2.91 | 1.42–5.94 | 0.003 | 2.62 | 1.47–4.69 | 0.001 |
| LnsCD163 | 0.70 | 0.17–2.92 | 0.63 | — | — | — |
| LnsMR | — | — | — | 1.64 | 0.27–10.13 | 0.59 |
| Combined cohortc | sCD163, ELF, and AOM | sMR, ELF, and AOM | ||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| AOM, per point increase | 1.65 | 1.15–2.38 | 0.007 | 1.65 | 1.15–2.37 | 0.007 |
| ELF test, per point increase | 1.66 | 1.31–2.10 | <0.001 | 1.66 | 1.35–2.04 | <0.001 |
| LnsCD163 | 0.92 | 0.52–1.63 | 0.78 | — | — | — |
| LnsMR | — | — | — | 0.89 | 0.50–1.60 | 0.70 |
| Center (Oslo = reference) | 0.64 | 0.33–1.25 | 0.19 | 0.66 | 0.34–1.27 | 0.21 |
Calculated in a subset of 128 patients with available AOM, events n = 60.
Calculated in a subset of 64 patients with available AOM, events n = 12.
Calculated in a subset of 192 patients with available AOM, events n = 72.
AOM, Amsterdam-Oxford Model for primary sclerosing cholangitis; CI, confidence interval; HR, hazard ratio; LnsCD163, logarithmic transformed soluble CD163; LnsMR, logarithmic transformed soluble mannose receptor; PSC, primary sclerosing cholangitis; sCD163, soluble CD163; sMR, soluble mannose receptor.