Figure 2. Ketamine evokes sustained cortical disinhibition, which is reversed with exogenous NRG1.
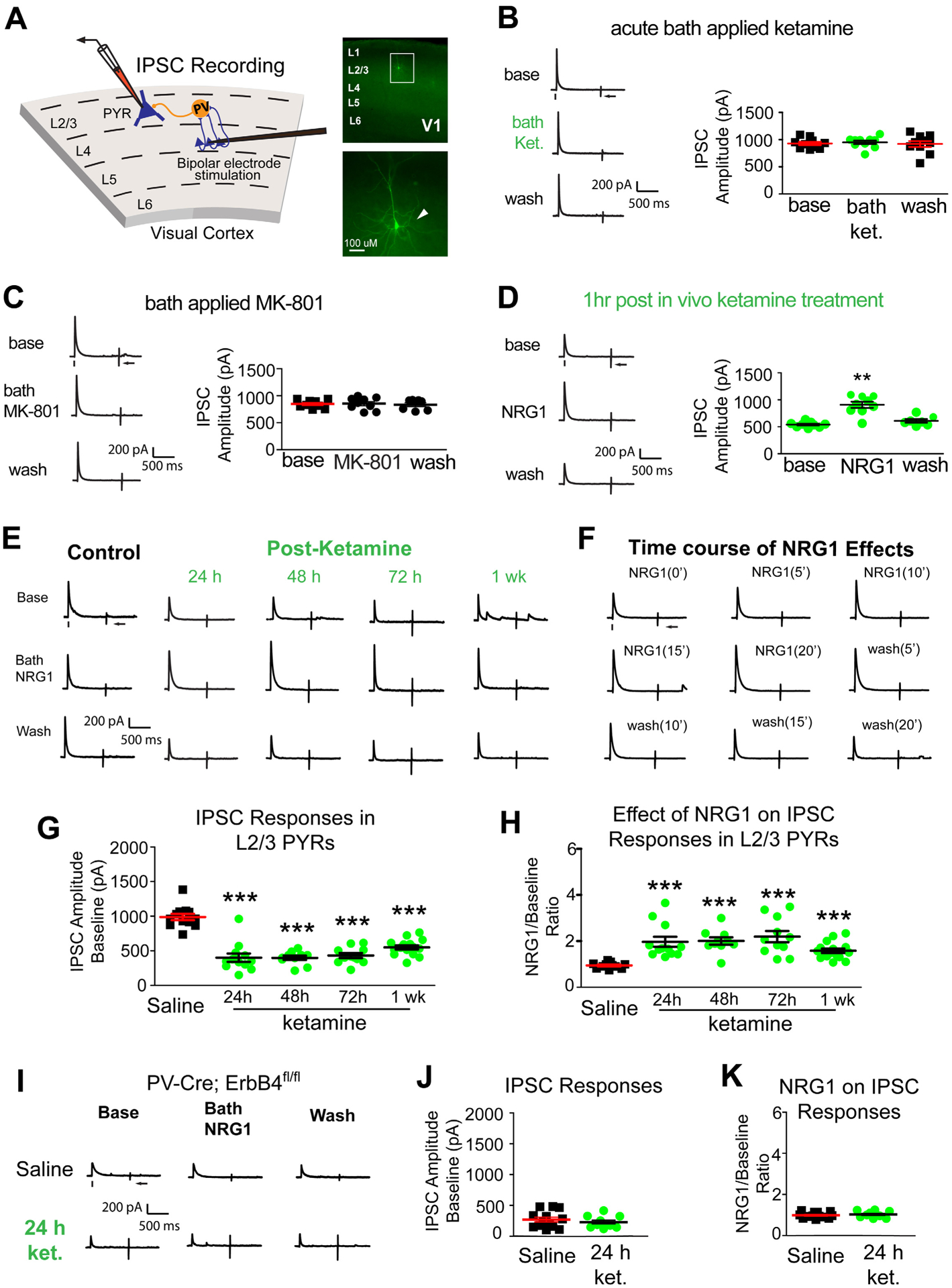
(A) Recording inhibitory postsynaptic currents (IPSCs) in L2/3 pyramidal (PYR) neurons by preferentially activating L4→L2/3 feedforward projections to L2/3 PV neurons through L4 electrical stimulation. Recorded neurons are filled with biocytin for post-hoc confirmation. (B-F) Recordings maintained on the same neuron during baseline, treatment, and washout. For each trial, electrical stimulation (1 ms, 20 μA) was applied, represented by a black tick beneath one example trace. The arrow indicates the current injection response to monitor access resistance during the experiment. (B) Acute bath application of ketamine does not induce any change in inhibitory inputs to L2/3 pyramidal neurons (n=10 same cells for control, drug application, and washout) in adult mouse visual cortex (P56 and above) (Friedman’s test: overall, p = 0.200). The data were obtained from 10 slices (1 cell per slice) of 5 different mice. (C) Acute bath application of MK-801 does not induce any change in inhibitory inputs to L2/3 pyramidal neurons [n=9 cells (7 cells have washout data) from 9 slices of 3 mice] (Friedman’s test: overall, p = 0.600). (D) Ketamine in vivo (10 mg/kg; s.c.) only 1 hour before recording reduces synaptic inhibition to L2/3 pyramidal cells. Bath application of NRG1 (5 nM) then reversed the effect and increased inhibitory inputs [n=9 cells (7 cells have washout data) from 9 slices of 3 mice]. (Friedman’s test: overall, p = 0.0008; Wilcoxon Signed-Rank test (adjusted for multiple comparisons): for NRG1 vs base, p = 0.0078; mean ± SEM). (E, G-K) Recordings are from different neurons and different mice at time points that are on different days (24, 48, 72, 1 week). (E) Ketamine in vivo (10 mg/kg; s.c.) reduces evoked IPSCs to PYR neurons at 24 hours (n=12 cells), 48 hours (n=10 cells), 72 hours (n=11 cells), and 1 week (n=12 cells). Ketamine evoked decreases in IPSCs are reversed with bath NRG1. (F) Bath NRG1 increases inhibitory inputs to L2/3 pyramidal neurons with 10–20 minutes and this is washed out within 20 minutes. (G,H) We recorded L2/3 pyramidal neurons: control saline (n=12 cells from 12 slices of 5 mice), 24 hr [n=12 cells (9 cells have washout data) from 12 slices of 9 mice], 48 hr [n=10 cells (all cells have washout data) from 10 slices of 4 mice], 72 hr [n=11 cells (9 cells have washout data) from 11 slices of 5 mice], and 1 week [n=15 cells (13 cells have washout data) from 15 slices of 8 mice] before and after bath NRG1 application. (G) Compared with saline IPSC amplitudes are significantly reduced after in vivo ketamine treatment (Kruskal-Wallis test: overall p = 4.8 × 10−7. Mann-Whitney U test (adjusted for multiple comparisons): in vivo ketamine vs saline, 24h p = 0.0006, 48h p = 0.0004, 72h p= 0.0002, 1wk p = 6.29 × 10−5; mean ± SEM]. (H) NRG1 increases average evoked IPSC amplitudes ratios in L2/3 pyramidal neurons (control saline (n=12 cells), 24 (n=12 cells), 48 (n=10 cells) and 72 hours (n=11 cells) and 1 week (n=15 cells) after NRG1 (Kruskal-Wallis test: overall, p = 1.8×10−6. Mann-Whitney U -test (adjusted for multiple comparisons): in vivo ketamine vs saline, 24h p = 0.0002, 48h p = 0.0006, 72h p = 0.0002, 1wk p = 0.0001; mean ± SEM). (I-K) Removal of ErbB4 in PVs prevents ketamine-induced decreases in IPSCs in L2/3 excitatory neurons: neither ketamine (10mg/kg; s.c.) nor NRG1 affects evoked IPSCs to L2/3 pyramidal neurons of PV-Cre; ErbB4fl/fl mice. Representative IPSC traces shown in (I), and summarized in (J, K) (Figure 2J. Mann-Whitney U test: p = 0.5149) (Figure 2K. Mann-Whitney U test: p = 0.4757). Also see related information in Table S1.
