Figure 5. HNK evokes sustained reduction of cortical inhibition and loss of excitatory inputs to PV inhibitory neurons, both of which are restored with NRG1.
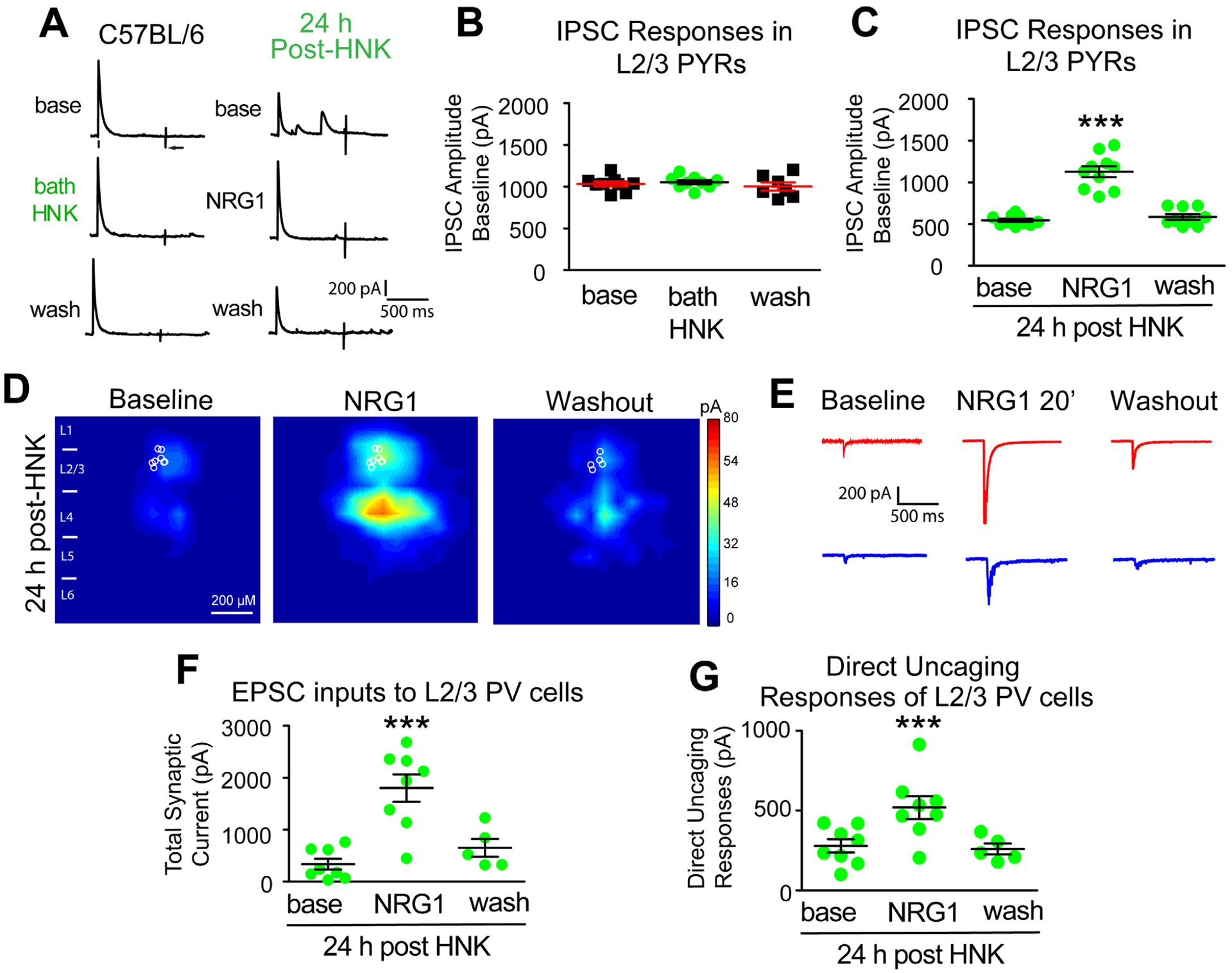
(A-G) Recordings maintained on the same neuron during baseline, treatment, and washout. (A-B) Acute bath application of (2R, 6R)-HNK (100 μM) does not change evoked IPSC strength to L2/3 excitatory pyramidals (n=8 cells). IPSCs were recorded in L2/3 pyramidal (PYR) neurons through L4 electrical stimulation as shown in Figure 2A. (C) HNK treatment (10 mg/kg; s.c.) at 24 hours before recording induces a reduction of IPSCs to L2/3 pyramidals. NRG1 (5 nM) reversed the reduced inhibition induced by HNK treatment (n=10 cells) (Figure 5A,C. Friedman’s test: overall, p = 0.0007; Wilcoxon Signed-Rank test (adjusted for multiple comparisons): for NRG1 vs base, p = 0.0039); mean ± SEM). (D) LSPS mapping of local excitatory synaptic inputs to PVs. (E) Illustration of example responses of a PV neuron in HNK treated mouse cortex with LSPS in a perisomatic location (red traces) and in a presynaptic input site (blue traces) at baseline, NRG1 for 20 minutes and washout. (F-G) Both total synaptic inputs (n=8 cells) and direct uncaging (n=8 cells) response are reduced 24 hours after HNK treatment in vivo. NRG1 (5 nM) significantly restores both total excitatory synaptic inputs and direct excitatory synaptic inputs onto PVs in V1 of HNK mice (Figure 5F. Friedman’s test: overall, p = 0.0034; Wilcoxon Signed-Rank test (adjusted for multiple comparisons): for NRG1 vs base, p = 0.0156; mean ± SEM) (Figure 5G. Friedman’s test: overall, p = 0.0052; Wilcoxon Signed-Rank test (adjusted for multiple comparisons): for NRG1 vs base, p = 0.0156; mean ± SEM). Also see related information in Table S1.
