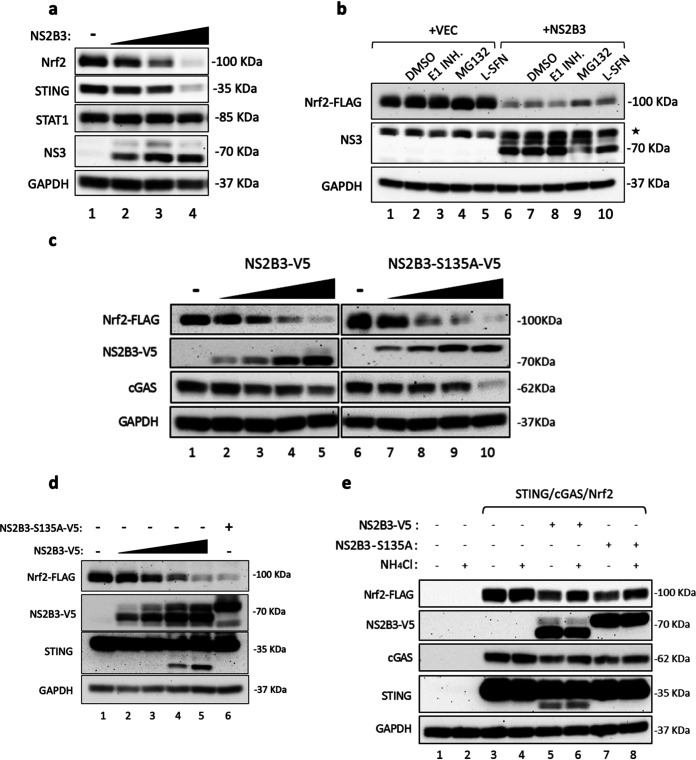
FIG 6.
The DENV NS2B3 protease complex mediates Nrf2 degradation. (a) 293T cells were cotransfected with human Nrf2, STING, and different amounts of an NS2B3 plasmid (0.5, 1, and 2 μg). Protein expression was analyzed at 24 h by Western blotting using anti-Nrf2, anti-STING, and anti-NS3 antibodies. An anti-STAT1 antibody was used to detect endogenous STAT1 protein. GAPDH was used as a loading control. (b) Coexpression of a human Nrf2-FLAG plasmid (1 μg) and WT DENV NS2B3 (2 μg) or an empty vector (VEC). At 24 h after transfection, cells were treated for 24 h with either DMSO, the E1 ubiquitin enzyme PYR41 (1 μM), or the proteasome inhibitor MG132 (5 μM). Protein expression was analyzed by Western blotting using anti- FLAG, anti-NS3, and anti-GAPDH antibodies. The asterisk indicates a nonspecific band. (c) HEK293T cells were cotransfected with human Nrf2-FLAG (1 μg), cGAS (150 ng), and different amounts of the wild-type DENV NS2B3-V5 plasmid or the protease-dead mutant DENV NS2B3-S135A-V5 plasmid (0, 1, 2, 4, and 10 μg). Cells were harvested 24 h after transfection, and protein expression was analyzed by Western blotting using anti-FLAG, anti-V5, anti-cGAS, and anti-GAPDH antibodies. (d) Coexpression of Nrf2-FLAG and different amounts of WT DENV NS2B3-V5 (0.5, 1, 2, and 5 μg) or NS2B3-S135A-V5 (5 μg) analyzed at 24 h by Western blotting using anti-FLAG, anti-V5, and anti-GAPDH antibodies. (e) 293T cells were cotransfected with human Nrf2 (1 μg), STING (150 ng), cGAS (150 ng), and either WT DENV NS2B3-V5 (2 μg) or NS2B3–S135A-V5 (2 μg). At 16 h after transfection, cells were either left untreated or treated for 8 h with NH4Cl (10 mM). Analysis by Western blotting was performed using anti-FLAG, anti-V5, anti-STING, anti-cGAS, and anti-GAPDH antibodies. Results are representative of three independent experiments.