Abstract
Aims
Aneurysmal bone cysts (ABCs) are locally aggressive lesions typically found in the long bones of children and adolescents. A variety of management strategies have been reported to be effective in the treatment of these lesions. The purpose of this review was to assess the effectiveness of current strategies for the management of primary ABCs of the long bones.
Methods
A systematic review of the published literature was performed to identify all articles relating to the management of primary ABCs. Studies required a minimum 12-month follow-up and case series reporting on under ten participants were not included.
Results
A total of 28 articles meeting the eligibility criteria were included in this review, and all but one were retrospective in design. Due to heterogeneity in study design, treatment, and outcome reporting, data synthesis and group comparison was not possible. The most common treatment option reported on was surgical curettage with or without a form of adjuvant therapy, followed by injection-based therapies. Of the 594 patients treated with curettage across 17 studies, 86 (14.4%) failed to heal or experienced a recurrence. Similar outcomes were reported for 57 (14.70%) of the 387 patients treated with injection therapy across 12 studies. Only one study directly compared curettage with injection therapy (polidocanol), randomizing 94 patients into both treatment groups. This study was at risk of bias and provided low-quality evidence of a lack of difference between the two interventions, reporting success rates of 93.3% and 84.8% for injection and surgical treatment groups, respectively.
Conclusion
While both surgery and sclerotherapy are widely implemented for treatment of ABCs, there is currently no good quality evidence to support the use of one option over the other. There is a need for prospective multicentre randomized controlled trials (RCTs) on interventions for the treatment of ABCs.
Cite this article: Bone Jt Open 2021;2(2):125–133.
Keywords: Aneurysmal bone cyst, Long bones, Curettage, Infection, Sclerotherapy
Introduction
Aneurysmal bone cysts (ABCs) are non-malignant, blood-filled, tumour-like lesions that most commonly occur in the metaphysis of long bones and the vertebrae. These lesions have a prevalence of 1.4 cases per 100,000, with 75% to 90% occurring in patients under the age of 20 years.1,2 Genetic studies have confirmed that a subset of ABCs are primary neoplastic lesions, defined by a specific translocation. These primary ABCs are thought to make up 50% to 70% of cases.3 The remaining cases are secondary to haemorrhagic degenerative events in pre-existing bone lesions, including giant cell tumours, unicameral bone cysts, osteoblastomas, and chondroblastomas.2
Patients may present with an insidious onset of pain, associated swelling, deformity, and in some cases a pathological fracture. Plain radiographs demonstrate a cystic lesion with thin sclerotic margins, and changes in local bony anatomy due to expansion of the cyst may also be present. MRI can identify features more specific to ABCs, such as septa within the lesion and variably aged blood contained within the cystic cavities.4 Histological analysis demonstrates the osseous septa separating the blood-filled cavities to be lined with fibroblasts and osteoclastic giant cells.5
Treatment is largely aimed at promoting cyst healing, thereby reducing pain and risk of pathological fracture. Few advocate for a watch-and-wait approach, adopted on the basis of evidence of some cysts healing without treatment6 or following biopsy.7,8 However, the mainstay of management has traditionally been intralesional curettage with or without bone grafting.2,9-14 A variety of adjuvant treatments can be applied alongside curettage with a view to minimizing recurrence, including phenol, cryotherapy, and argon beam coagulation.13,15-17 The more aggressive surgical option of en bloc resection is thought to offer a lower risk of recurrence, but may require complex reconstruction, and is not always feasible considering the size and location of the lesion.17,18 Intralesional injection techniques are available as an alternative to surgery and offer the benefits of being minimally invasive and potentially more cost-effective.14,19 Injectable substances reportedly used for ABC management include alcohol, polidocanol, and Ethibloc (Ethnor Laboratories, Ethicon, Germany).19-21 Several other treatment strategies reported in the literature include selective arterial embolization,22 systemic therapy with receptor activator of nuclear factor kappa-Β ligand (RANKL) inhibitors (Denosumab),23 and radiation therapy.24
Currently there is no consensus on the optimal management of ABCs. Risk of complications and potential for recurrence need to be balanced against the invasiveness of an intervention when considering treatment options. The purpose of this review was to assess the efficacy of interventions for treating primary ABCs in the long bones of children and adults.
Methods
Search strategy
A comprehensive electronic search of the MEDLINE database was performed using the PubMed search engine. A search was also conducted on the Cochrane Central Register of Controlled Trials (CENTRAL) using the website-specific search engine. Databases were searched on 15 October 2020 and restricted to articles published from 1 January 2000. Reference lists from all relevant studies were manually reviewed. A detailed report of the search strategy is presented in Supplementary Material.
Eligibility criteria
We intended to include randomized controlled trials (RCTs), quasi-randomized controlled trials, prospective cohort studies, retrospective case-control studies, and case series evaluating all methods for treating ABCs. Case series with fewer than ten patients were not included to reduce the risk of reporting bias. Only articles published in English were considered for review. The target population was children and adults of all ages with primary ABCs located within long bones. Studies reporting on lesions in the axial skeleton were considered if long bone data accounted for greater than 50% of cases. Studies involving patients with secondary or extraosseous ABCs were not included. Primary outcome measures considered for this review were cyst healing25 and recurrence. Other outcome measures included cyst volume reduction, pain scores, validated functional outcome measures (e.g. Musculoskeletal Tumour Society Score, Paediatric Orthopaedics Society of North America instruments),26,27 and complications. For the primary outcome measures a minimum of 12 months follow-up was required for study inclusion.
Data collection and extraction
Two authors (LB and AW) independently screened the titles, abstracts, and keywords of every article retrieved via the search strategy. Decisions on inclusion were made according to the pre-stated eligibility criteria. Full texts were obtained for studies that fulfilled the inclusion criteria and for studies where there was uncertainly around eligibility. Two authors (LB and AW) independently extracted study details and data for each included study using an electronic data collection form. Disagreement was resolved by consensus or consultation with the third author (AK). The Cochrane Risk of Bias tool was used to assess the methodological quality of any randomized trials.28 The validated tool designed by Moga et al29 was used to assess the quality of case series. The Preferred Reporting Item for Systematic Reviews and Meta-analysis (PRISMA) was used to report the findings (Checklist is provided in Supplementary Material).
Results
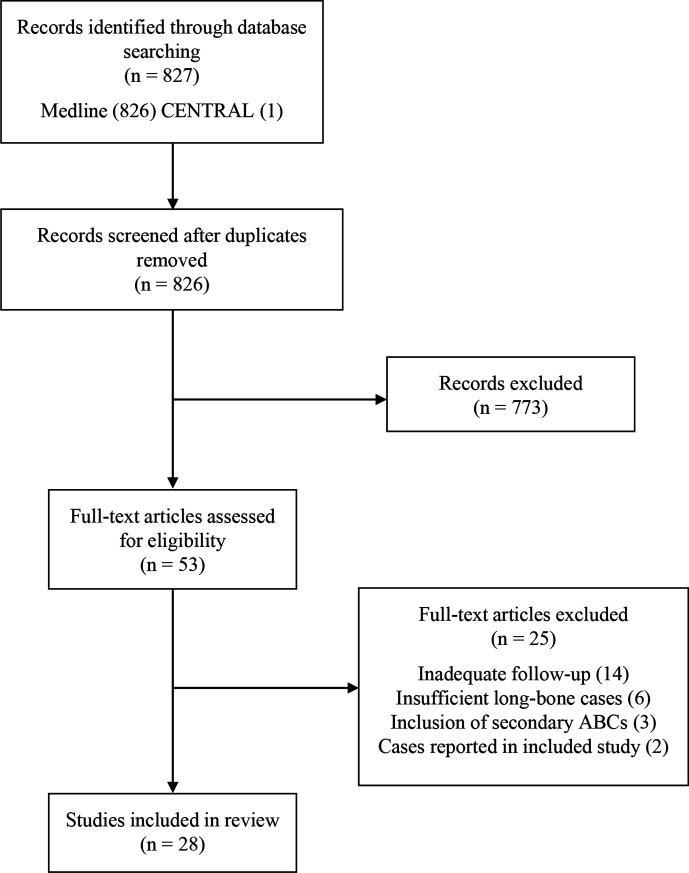
The electronic literature search returned 826 citations of potential studies related to the management of primary ABCs and 28 studies were included for review (Figure 1). The majority of included studies were retrospective case series. Four retrospective cohort studies investigating differences between alternative surgical approaches17,18,30,31 and one RCT comparing curettage to percutaneous sclerotherapy were included.20 A total of 18 studies reported on surgical treatment methods11,13,16-18,20,30-41 and 12 studies reported on percutaneous injection-based therapies.8,19,20,42-50 One included study reported outcomes for two subjects treated with radiation therapy.31
Fig. 1.
A flowchart detailing the search strategy, results of the literature search, and study selection process.
In total, outcome data were available on 1,056 cysts over 1,055 patients, with a mean study follow-up ranging from 29 to 110 months. Patient age across all studies ranged from one to 66 years old with mean study age ranging from eight to 26 years old. The male to female distribution was 583:456 for all cases where sex was reported (Table I). In all, 77% of cysts were located in the long bones with the humerus being the most common site (25%), followed by femur (24%), tibia (15%), fibula (7%), and forearm (6%) (Table II). Of all cases, 182 (17%) were reported to have presented with a pathological fracture, and 70 (7%) cysts were reported to have undergone previous treatment. Treatment success was often determined based on a radiological classification of cyst healing (e.g. modified Neer classification).25 However, healing and recurrence were not clearly defined in all studies. It was also noted that the term recurrence was occasionally used to refer to persistence of cyst, rather than reappearance after successful initial treatment. To ensure consistent reporting of outcomes, recurrences in these cases were reported as treatment failures (Tables III to VI).
Table I.
Articles included in this review, including patient characteristics.
| Author | Year | Study design | Patients, n | Mean age, yrs (range) | M:F | % long bones |
|---|---|---|---|---|---|---|
| Puri | 2020 | Case series | 55 | 20 (1 to 54) | 26:29 | 67 |
| Marie-Hardy | 2019 | Case series | 54 | 10 (1 to 15) | 32:22 | 84 |
| Aiba | 2018 | Case series | 30 | 17 (6 to 40) | 18:12 | 60 |
| Oliveira | 2018 | Case series | 47 | 18 (4 to 54) | 23:24 | 64 |
| Rahman | 2018 | Case series | 16 | 15 (7 to 32) | 10:6 | 100 |
| Ghanem | 2017 | Case series | 16 | 10 (5 to 14) | 10:6 | 75 |
| Mostafa | 2017 | Case series | 15 | 12 (6 to 16) | 12:3 | 100 |
| Shiels | 2016 | Case series | 16 | 7 (2 to 15) | 100 | |
| Crowe | 2015 | Case series | 11 | 19 (2 to 47) | 7:4 | 64 |
| Erol | 2015 | Case series | 64 | 10 (5 to 18)* | 38:26 | 83 |
| Kececi | 2014 | Retrospective cohort | 85 | 18 (3 to 66) | 45:40 | 71 |
| Wang | 2014 | Case series | 31 | 26 (8 to 62) | 15:16 | 74 |
| Flont | 2013 | Retrospective cohort | 26 | 13 (2 to 18) | 11:15 | 85 |
| Ibrahim | 2012 | Retrospective cohort | 17 | 12 (2 to 18) | 7:10 | 82 |
| Cummings | 2010 | Case series | 29 | 12 (4 to 39) | 22:7 | 76 |
| Docquier | 2010 | Case series | 21 | 16 (8 to 28) | 11:10 | 52 |
| George | 2009 | Case series | 31 | (3 to 16)† | 19:14‡ | 97 |
| Peeters | 2009 | Case series | 80 | 17 (3 to 52) | 45:35 | 79 |
| Varshney | 2009 | RCT | 91 | 21, 27§ | 61:30 | 86 |
| Lin | 2008 | Case series | 53 | 14 (4 to 65) | 25:28 | 68 |
| Basarir | 2007 | Retrospective cohort | 56 | 11 (2 to 16) | 33:23 | 71 |
| Rastogi | 2006 | Case series | 72 | 16 (3 to 38) | 46:26 | 86 |
| Cottalorda | 2005 | Case series | 21 | 4 (1.5 to 5) | 14:7 | 81 |
| de Gauzy | 2005 | Case series | 12 | 10 (5 to 14)* | 9:3 | 83 |
| Dormans | 2004 | Case series | 45 | 12 (2 to 18) | 23:22 | 64 |
| Topouchian | 2004 | Case series | 15 | 10 (3 to 15) | 8:7 | 73 |
| Adamsbaum | 2003 | Case series | 17 | 8 (2 to 18) | 5:12 | 71 |
| Ramirez | 2002 | Case series | 29 | 10 (1 to 20)* | 9:20 | 62 |
Median age (interquartile range).
Range only, no mean age provided.
Two lost to follow-up: information on sex and anatomical site not available.
Two values, no range provided.
RCT, randomized controlled trial.
Table II.
Anatomical location of all treated cysts, including additional two cysts lost to follow-up with unknown location.
| Anatomical location | Number of cysts | % of total |
|---|---|---|
| Humerus | 260 | 25 |
| Radius | 30 | 3 |
| Ulna | 14 | < 1 |
| Forearm (bone not specified) | 15 | < 1 |
| Hand | 25 | 2 |
| Femur | 253 | 24 |
| Tibia | 159 | 15 |
| Fibula | 79 | 7 |
| Foot | 45 | 4 |
| Hand and foot (not specified) | 4 | < 1 |
| Spine | 23 | 2 |
| Pelvis | 102 | 10 |
| Scapula | 7 | < 1 |
| Clavicle | 38 | 4 |
| Rib | 1 | < 1 |
| Unknown location | 3 | < 1 |
| Total | 1,058 |
Table III.
Outcome summary for all surgical treatments.
| Study | Mean follow-up, mths (range) |
Primary treatment | Cysts | Failure (%) |
Recurrence (%) |
|---|---|---|---|---|---|
| Aiba 2018 |
55 (16 to 149) | Endoscopic curettage | 30 | 9 (30) | 3 (10) |
| Rahman 2018 |
51 (24 to 78) | Curettage + burr + cryosurgery + bone graft | 16 | 1 (6) | |
| Mostafa 2017 |
45 (24 to 68) | Curettage + argon beam coagulation + bone graft | 15 | 1 (7) | |
| Crowe 2015 |
29 (13 to 56) | Curettage ± burr ± phenol ± argon beam ± H2O2 + bone graft | 10 | 1 (10) | |
| En bloc resection + bone graft + internal fixation | 1 | 0 (0) | |||
| Erol 2015 |
66 (28 to 130)* | Curettage + burr ± bone graft ± PMMA ± internal fixation ± preoperative embolization | 59 | 2 (3) | 2 (3) |
| En bloc resection ± bone graft ± endoprosthetic reconstruction ± internal fixation | 5 | 0 (0) | 0 (0) | ||
| Kececi 2014 |
108 (48 to 300) | Curettage ± bone graft ± PMMA | 14 | 1 (7) | |
| Curettage + burr ± bone graft ± PMMA | 19 | 3 (16) | |||
| Curettage + burr + alcohol + phenol ± bone graft ± PMMA | 43 | 6 (14) | |||
| En bloc resection | 9 | 0 (0) | |||
| Wang 2014 |
84 (24 to 216) | Curettage + burr ± internal fixation | 31 | 1 (3) | |
| Flont 2013 |
110 (36 to 228) | Curettage + burr ± bone graft | 16 | 2 (13) | |
| En bloc resection ± bone graft | 10 | 0 (0) | |||
| Ibrahim 2012 |
40 (24 to 70) | Percutaneous curettage and suction | 9 | 1 (11) | 1 (11) |
| Curettage + burr ± bone graft ± internal fixation | 8 | 1 (13) | 0 (0) | ||
| Cummings 2010 |
39 (19 to 88) | Curettage + burr + argon beam ± bone graft | 17 | 0 (0) | |
| Curettage + burr ± phenol ± bone graft | 12 | 4 (33) | |||
| Docquier 2010 |
53 (24 to 106) | Open biopsy + DBP ± BM ± TPC | 21 | 5 (24) | |
| Peeters 2009 |
55 (24 to 122) | Curettage + burr + cryosurgery ± bone grafting ± internal fixation | 80 | 4 (5) | |
| Lin 2008 |
35 (24 to 112) | Curettage ± burr + bone graft | 53 | 10 (19) | |
| Basarir 2007 |
48 (24 to 194) | Curettage + bone graft | 23 | 6 (26) | |
| En bloc resection | 19 | 0 (0) | |||
| Radiation therapy | 2 | 1 (50) | |||
| Curettage ± cauterization ± PMMA + bone graft | 12 | 2 (17) | |||
| Dormans 2004 |
46 (24 to 99) | Curettage ± burr ± caterization ± phenol ± H2O2 ± preoperative embolization±bone graft | 44 | 8 (18) | |
| En bloc resection | 1 | 0 (0) | |||
| Ramirez 2002 |
62 (26 to 117) | Curettage + bone graft | 23 | 8 (35) | |
| En bloc resection ± bone graft ± spinal fusion | 6 | 0 (0) |
“±” here designates “with or without”.
Median follow-up (interquartile range).
BM, bone marrow; DBP, demineralized bone powder; PMMA, polymethyl methacrylate; TPC, tricalcium phosphate cylinder.
Table VI.
Number of reported complications.
| Surgery (n = 667) | Number |
|---|---|
| Growth deformity | 33 |
| Superficial infection | 8 |
| Deep infection | 7 |
| Transient neurology | 5 |
| Pathological fracture | 5 |
| Persistent pain | 4 |
| Reduced joint ROM | 3 |
| Graft nonunion | 1 |
| Injection therapy (n = 387 ) | |
| Local induration | 55 |
| Local inflammation | 35 |
| Hypopigmentation | 14 |
| Aseptic fistula | 7 |
| Growth deformity | 6 |
| Pathological fracture | 5 |
| Extravasion of contrast/sclerosant | 4 |
| Deep infection | 2 |
| Dizziness | 2 |
| Pulmonary embolism | 1 |
| Local skin necrosis | 1 |
| Sterile abscess | 1 |
| Persistent pain | 1 |
| Bradycardia | 1 |
ROM, range of motion.
Table V.
Outcome summary for studies evaluating both surgical and injection-based treatments.
| Study | Mean follow-up, mths (range) |
Primary treatment | Mean number of injections (range) | Cysts, n | Failure, n (%) | Recurrence, n (%) |
|---|---|---|---|---|---|---|
| Varshney 2009 |
53 (38 to 73) | Polidocanol injections | 2.3 (1 to 5) | 45 | 1 (2) | 2 (4) |
| Curettage+ burr + bone graft | 46 | 0 (0) | 7 (15) | |||
| Cottalorda 2005 |
64 (25 to 169) | Ethibloc injection | 1 | 4 | 1 (25) | |
| Methylprednisolone injection | 1 | 2 | 2 (100) | |||
| Curettage±bone graft | 14 | 2 (14) | ||||
| En bloc resection | 1 | 0 (0) |
Surgical treatment mostly consisted of curettage with or without mechanical burring,11,13,16-18,20,30,33,35,37,41 bone/bone substitute grafting,11,13,16-18,20,30,31,33,34,36-39,41 cauterization,31,38 argon beam coagulation,16,33,34 phenol,16,17,33,38 polymethyl methacrylate (PMMA),11,17,31 cryosurgery,13,41 hydrogen peroxide,33,38 preoperative embolization,11,38 and internal fixation.11,13,30,35 Treatment heterogeneity was often observed with a variety of different adjuvant treatments being used within a single series. For the 594 patients who underwent curettage (with or without adjuvant therapy), 86 (14.4%) failed to heal or experienced a recurrence, with individual study combined failure and recurrence rates ranging from 0% to 40%. A total of 52 cysts across eight studies had undergone en bloc resection and healed successfully with no recurrence.11,17,18,31,33,36,38,39 Two studies of minimally invasive curettage techniques, without adjuvant therapy or grafting, reported treatment failures or recurrence in 40% (12/30) and 22% (2/9) of cases.30,32 One study evaluated open biopsy with application of a paste composed of demineralized bone and bone marrow aspirate, without any formal curettage; 24% (5/21) of patients failed to heal at a mean follow-up of 53 (24 to 106) months.
The most commonly injected substance was Polidocanol (44%);19,46 other substances included Ethibloc (20%),39,45,47-49 alcohol (14%),42 calcitonin with methylprednisolone (12%),43 methylprednisolone alone (1%),39 doxycycline (4%),50 and Surgiflo with alcohol (4%).44 The mean number of injections delivered to patients in each study varied from 1.1 to 6.4, with patients in five studies receiving five or more injections.19,20,43,46,50 Patients receiving doxycycline injections required the greatest number of treatment sessions, ranging between two and 14 injections.50 The time interval between repeat injections also varied, with one study reporting intervals ranging between 0.2 and 20.1 months.42 Indications for repeat injection were not reliably reported and the stage at which treatment would have been considered a failure was often unclear. For the 387 cysts treated with injection therapy, 57 (14.7%) failed to heal or recurred following a period of healing.
We found only one study comparing injection-based therapy with surgical treatment.20 This single-centre RCT was not sufficiently powered to detect significant differences with healing as the primary outcome measure, with 94 patients randomized to two groups and three lost to follow-up. The study was also found to be at significant risk of bias due to non-stratified randomization, resulting in different baseline characteristics of treatment groups, and a lack of assessor blinding. The authors used objective radiological criteria for determining cyst healing and clearly define recurrence as appearance of new radiolucency in a previously opacified cyst. Treatment completion for injection therapy was also clearly defined based on radiological progression. No statistically significant differences were noted in overall treatment success between the injection and surgical treatment groups (93.3% vs 84.8%), however, surgery was found to result in poorer functional outcomes and a higher number of clinically important complications, including two deep infections and two disturbances of growth.
The complication profile of each treatment modality was not consistently reported across all studies. Table IV (Table VI) presents the overall frequency of specific complications within all injection and surgery treatment groups included in the review. Growth deformity (limb length discrepancy or angular plane deformity) was the most common complication reported in the surgical treatment groups. Local skin related complications (induration, inflammation, hypopigmentation) were most common among the injection therapy groups. Two studies investigating Ethibloc injections reported several serious complications including aseptic fistulae and one pulmonary embolism.48,49
Table IV.
Outcome summary for all injection-based treatments.
| Study | Mean follow-up, mths (range) |
Primary treatment | Mean number of injections (range) | Cysts, n | Failure, n (%) | Recurrence, n (%) |
|---|---|---|---|---|---|---|
| Puri 2020 |
62 (20 to 111) | Polidocanol | 2.0 (1 to 5) | 55 | 9 (16) | 4 (7) |
| Marie-Hardy 2019 |
51 (16 to 117) | Alcohol | 1.7 (1 to 4) | 55 | 9 (16) | |
| Oliveira 2018 |
46 (24-?) | Calcitonin+ methylprednisolone | 2.8 (1 to 7) | 47 | 4 (9) | 5 (11) |
| Ghanem 2017 |
36 (24 to 71) | Surgiflo + alcohol | 1.1 (1 to 2) | 16 | 5 (31) | |
| Shiels 2016 |
42 (24 to 106) | Doxycyline | 6.4 (2 to 14) | 16 | 0 (0) | 1 (6) |
| George 2009 |
54 (22 to 90) | Ethibloc | 1.2 (1 to 2) | 31 | 2 (6) | |
| Rastogi 2006 |
34 (27 to 80) | Polidocanol | 3.0 (1 to 5) | 72 | 2 (3) | |
| de Gauzy 2005 |
61 (24 to 80) | Ethibloc | 1.1 (1 to 2) | 12 | 3 (25) | |
| Topouchian 2004 |
80 (47 to 116) | Ethibloc | 1.6 (1 to 3) | 15 | 4 (27) | |
| Adamsbaum 2003 |
60 (18 to 132) | Ethibloc | 1.4 (1 to 3) | 17 | 3 (18) |
N/A, not available
Discussion
This systematic review has critically evaluated studies pertaining to the management of primary ABCs of the long bones. Given the heterogeneity in study design, treatment techniques, and reporting, it was not possible to perform a quantitative synthesis of the outcome data. All but one study was retrospective in design and only nine studies reported on sample sizes of more than 50 patients. Treatment protocols were rarely consistent within individual series; multiple adjuvant therapies were used alongside surgical curettage and a variable number of treatments sessions were performed for patients receiving injection therapy. Indications for adjuvant treatments or repeat injection therapy were often unclear, and treatment success was poorly defined in many studies. The only identified prospective study was found to be at risk of bias and not sufficiently powered to detect significant differences in primary outcomes. While both surgery and sclerotherapy are widely implemented for treatment of ABCs, there is currently no good quality evidence to support the use of one option over the other.
This review focused on ABCs located in the long bones, as lesions in other anatomical areas, including the spine and flat bones, can present unique challenges and require individualized treatment options. Series reporting on fewer than ten patients were excluded to reduce risk of reporting bias, and minimum follow-up was restricted to 12 months to ensure an appropriate length of time was allowed to detect the majority of recurrences.12 Surgical curettage is reported to be the most commonly implemented treatment for ABCs2,43 and the majority of studies included in this review evaluated curettage with or without some form of adjuvant therapy and grafting. The remaining articles evaluated repetitive injection therapy and failure rates were found to be similar across both groups. As a result, several authors have concluded that injection-based therapy is potentially a superior treatment option when considering the invasiveness and complication profile of open surgery.19,20,42,43 With a predominantly paediatric population being affected, however, we must also consider the considerable burden associated with the multiple general anaesthetics required for most injection therapies. In addition to the disruption this can cause to a child’s life, there is also a risk that repeated general anaesthesia can cause emotional and behavioural disturbances.51
Several studies attempted to compare surgical methods and the benefits of particular adjuvant therapies, however given study design and small sample sizes, no strong conclusions can be drawn regarding the superiority of one particular adjuvant or treatment protocol. En bloc resection was studied in small numbers and it is worth noting that all reported cases healed with no evidence of recurrence. For injection therapies, the majority of injected substances were alcohol-based, with single studies evaluating doxycycline and calcitonin with methylprednisolone. It was not possible to determine the ideal injectable substance, optimal number of treatments, or time interval between treatments. Ethibloc injections appeared as effective as other options but in some cases were associated with serious complications.48 Since publication of the included studies, Ethibloc has been withdrawn from commercial use. Factors associated with treatment success or failure were examined in several studies, which identified younger age of presentation,17,31,32,37 involvement of the physis,32,37 and previous treatment (recurrent cysts)31 to be negative prognostic indicators. Pathological fracture was not investigated as a potential prognostic indicator.
ABCs are locally aggressive benign lesions often found in the long bones of children and young adults. Several treatment options have been proposed in the literature, which vary significantly in cost, patient burden, and complication profile. This review has failed to identify any controlled studies that support the use of one treatment strategy over another, and so there is a need for good-quality prospective multicentre RCTs on interventions for the treatment of ABCs. Future studies should implement standardized treatment protocols, objective definitions of treatment success and ideally a five-year follow-up.
Footnotes
Author contributions: L. Bavan: Performed the literature search, Conducted the eligibility screening, Extracted the data, Did the quality assessment, Edited the manuscript.
A. Wijendra: Performed the literature search, Conducted the eligibility screening, Did the quality assessment, Extracted the data.
A. Kothari: Conducted the eligibility screening, Did the quality assessment, Extracted the data, Edited the manuscript.
Funding statement: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Supplementary material: Medline search strategy (January 2000 to October 2020) and PRISMA checklist.
References
- 1. Mascard E, Gomez-Brouchet A, Lambot K. Bone cysts: unicameral and aneurysmal bone cyst. Orthop Traumatol Surg Res. 2015;101(1 Suppl):S119–S127. [DOI] [PubMed] [Google Scholar]
- 2. Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. J Am Acad Orthop Surg. 2012;20(4):233–241. [DOI] [PubMed] [Google Scholar]
- 3. Ye Y, Pringle LM, Lau AW, et al. . Tre17/Usp6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB. Oncogene. 2010;29(25):3619–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol. 1995;164(3):573–580. [DOI] [PubMed] [Google Scholar]
- 5. Czerniak B. Dorfman and Czerniak’s Bone Tumors. 2nd edn: Elsevier Health Sciences, 2015. [Google Scholar]
- 6. McQueen MM, Chalmers J, Smith GD. Spontaneous healing of aneurysmal bone cysts. A report of two cases. J Bone Joint Surg Br. 1985;67-B(2):310–312. [DOI] [PubMed] [Google Scholar]
- 7. Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26(3):615–625. [DOI] [PubMed] [Google Scholar]
- 8. Cottalorda J, Bourelle S. Modern concepts of primary aneurysmal bone cyst. Arch Orthop Trauma Surg. 2007;127(2):105–114. [DOI] [PubMed] [Google Scholar]
- 9. Reddy KIA, Sinnaeve F, Gaston CL, Grimer RJ, Carter SR. Aneurysmal bone cysts: do simple treatments work? Clin Orthop Relat Res. 2014;472(6):1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsagozis P, Brosjö O. Giant hydatid cyst of the pelvis, femur and retroperitoneal space: surgical treatment with extended hemipelvectomy. BMJ Case Rep. 2015;2015:bcr2015209715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erol B, Topkar MO, Caliskan E, Erbolukbas R. Surgical treatment of active or aggressive aneurysmal bone cysts in children. J Pediatr Orthop B. 2015;24(5):461–468. [DOI] [PubMed] [Google Scholar]
- 12. Hauschild O, Lüdemann M, Engelhardt M, et al. . Aneurysmal bone cyst (ABC) : treatment options and proposal of a follow-up regime. Acta Orthop Belg. 2016;82(3):474–483. [PubMed] [Google Scholar]
- 13. Peeters SP, Van der Geest ICM, de Rooy JWJ, Veth RPH, Schreuder HWB. Aneurysmal bone cyst: the role of cryosurgery as local adjuvant treatment. J Surg Oncol. 2009;100(8):719–724. [DOI] [PubMed] [Google Scholar]
- 14. Tsagozis P, Brosjö O. Current strategies for the treatment of aneurysmal bone cysts. Orthop Rev. 2015;7(4):6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park HY, Yang SK, Sheppard WL, et al. . Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med. 2016;9(4):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cummings JE, Smith RA, Heck RK. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keçeci B, Küçük L, Isayev A, Sabah D. Effect of adjuvant therapies on recurrence in aneurysmal bone cysts. Acta Orthop Traumatol Turc. 2014;48(5):500–506. [DOI] [PubMed] [Google Scholar]
- 18. Flont P, Kolacinska-Flont M, Niedzielski K. A comparison of cyst wall curettage and en bloc excision in the treatment of aneurysmal bone cysts. World J Surg Oncol. 2013;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Puri A, Hegde P, Gulia A, Parikh M. Primary aneurysmal bone cysts. Bone Joint J. 2020;102-B(2):186–190. [DOI] [PubMed] [Google Scholar]
- 20. Varshney MK, Rastogi S, Khan SA, Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin Orthop Relat Res. 2010;468(6):1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ulici A, Florea D-C, Carp M, Ladaru A, Tevanov I. Treatment of the aneurysmal bone cyst by percutaneous intracystic sclerotherapy using ethanol ninety five percent in children. Int Orthop. 2018;42(6):1413–1419. [DOI] [PubMed] [Google Scholar]
- 22. Rossi G, Mavrogenis AF, Facchini G, et al. . How effective is embolization with N-2-butyl-cyanoacrylate for aneurysmal bone cysts? Int Orthop. 2017;41(8):1685–1692. [DOI] [PubMed] [Google Scholar]
- 23. Dürr HR, Grahneis F, Baur-Melnyk A, et al. . Aneurysmal bone cyst: results of an off label treatment with denosumab. BMC Musculoskelet Disord. 2019;20(1):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu S, Hitchcock KE, Mendenhall WM. Radiation therapy for aneurysmal bone cysts. Am J Clin Oncol. 2017;40(6):621–624. [DOI] [PubMed] [Google Scholar]
- 25. Neer CS, Francis KC, Marcove RC, Terz J, Carbonara PN. Treatment of unicameral bone cyst. A follow-up study of one hundred seventy-five cases. J Bone Joint Surg Am. 1966;48-A(4):731–745. [PubMed] [Google Scholar]
- 26. Daltroy LH, Liang MH, Fossel AH, Goldberg MJ. The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. Pediatric Outcomes Instrument Development Group. Pediatric Orthopaedic Society of North America. J Pediatr Orthop. 1998;18(5):561–571. [DOI] [PubMed] [Google Scholar]
- 27. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–6. [PubMed] [Google Scholar]
- 28. Higgins JPT, Altman DG, Gøtzsche PC, et al. . The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moga C, Guo B, Schopflocher D, Harstall C. Development of a quality appraisal tool for case series studies using a modified Delphi technique. Institute of Health Economics, 2012. [Google Scholar]
- 30. Ibrahim T, Howard AW, Murnaghan ML, Hopyan S. Percutaneous curettage and suction for pediatric extremity aneurysmal bone cysts: is it adequate? J Pediatr Orthop. 2012;32(8):842–847. [DOI] [PubMed] [Google Scholar]
- 31. Başarir K, Pişkin A, Güçlü B, Yildiz Y, Sağlik Y. Aneurysmal bone cyst recurrence in children: a review of 56 patients. J Pediatr Orthop. 2007;27(8):938–943. [DOI] [PubMed] [Google Scholar]
- 32. Aiba H, Kobayashi M, Waguri-Nagaya Y, et al. . Treatment of aneurysmal bone cysts using endoscopic curettage. BMC Musculoskelet Disord. 2018;19(1):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crowe MM, Houdek MT, Moran SL, Kakar S. Aneurysmal bone cysts of the hand, wrist, and forearm. J Hand Surg Am. 2015;40(10):2052–2057. [DOI] [PubMed] [Google Scholar]
- 34. Mostafa MF, Abed YY, Fawzy SI. Shaped graft for aneurysmal bone cyst of upper limb bones. Strategies Trauma Limb Reconstr. 2017;12(3):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang EHM, Marfori ML, Serrano MVT, Rubio DA. Is curettage and high-speed burring sufficient treatment for aneurysmal bone cysts? Clin Orthop Relat Res. 2014;472(11):3483–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ramírez AR, Stanton RP. Aneurysmal bone cyst in 29 children. J Pediatr Orthop. 2002;22(4):533–539. [PubMed] [Google Scholar]
- 37. Lin PP, Brown C, Raymond AK, Deavers MT, Yasko AW. Aneurysmal bone cysts recur at juxtaphyseal locations in skeletally immature patients. Clin Orthop Relat Res. 2008;466(3):722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dormans JP, Hanna BG, Johnston DR, Khurana JS. Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin Orthop Relat Res. 2004;421:205–211. [DOI] [PubMed] [Google Scholar]
- 39. Cottalorda J, Kohler R, Chotel F, et al. . Recurrence of aneurysmal bone cysts in young children: a multicentre study. J Pediatr Orthop B. 2005;14(3):212–218. [DOI] [PubMed] [Google Scholar]
- 40. Docquier P-L, Delloye C. Treatment of aneurysmal bone cysts by introduction of demineralized bone and autogenous bone marrow. J Bone Joint Surg Am. 2005;87-A(10):2253–2258. [DOI] [PubMed] [Google Scholar]
- 41. Rahman MA, El Masry AM, Azmy SI. Review of 16 cases of aneurysmal bone cyst in the proximal femur treated by extended curettage and cryosurgery with reconstruction using autogenous nonvascularized fibula graft. J Orthop Surg. 2018;26(2):230949901878390. [DOI] [PubMed] [Google Scholar]
- 42. Marie-Hardy L, El Sayed L, Alves A, et al. . Percutaneous alcohol-based sclerotherapy in aneurysmal bone cyst in children and adolescents. Orthop Traumatol Surg Res. 2020;106(7):1313–1318. [DOI] [PubMed] [Google Scholar]
- 43. Oliveira MBDR, Meohas W, Silva RR, de Carvalho GS, Mello FCdeQ, Paschoal MEM. Percutaneous treatment of aneurysmal bone cyst with calcitonin and methylprednisolone. Acta Ortop Bras. 2018;26(5):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghanem I, Nicolas N, Rizkallah M, Slaba S. Sclerotherapy using Surgiflo and alcohol: a new alternative for the treatment of aneurysmal bone cysts. J Child Orthop. 2017;11(6):448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. George HL, Unnikrishnan PN, Garg NK, Sampath JS, Bass A, Bruce CE. Long-Term follow-up of Ethibloc injection in aneurysmal bone cysts. J Pediatr Orthop B. 2009;18(6):375–380. [DOI] [PubMed] [Google Scholar]
- 46. Rastogi S, Varshney MK, Trikha V, Khan SA, Choudhury B, Safaya R. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol. A review of 72 cases with long-term follow-up. J Bone Joint Surg Br. 2006;88-B(9):1212–1216. [DOI] [PubMed] [Google Scholar]
- 47. de Gauzy JS, Abid A, Accadbled F, Knorr G, Darodes P, Cahuzac JP. Percutaneous Ethibloc injection in the treatment of primary aneurysmal bone cysts. J Pediatr Orthop B. 2005;14(5):367–370. [DOI] [PubMed] [Google Scholar]
- 48. Topouchian V, Mazda K, Hamze B, Laredo J-D, Penneçot G-F. Aneurysmal bone cysts in children: complications of fibrosing agent injection. Radiology. 2004;232(2):522–526. [DOI] [PubMed] [Google Scholar]
- 49. Adamsbaum C, Mascard E, Guinebretière JM, Kalifa G, Dubousset J. Intralesional Ethibloc injections in primary aneurysmal bone cysts: an efficient and safe treatment. Skeletal Radiol. 2003;32(10):559–566. [DOI] [PubMed] [Google Scholar]
- 50. Shiels WE, Beebe AC, Mayerson JL. Percutaneous doxycycline treatment of Juxtaphyseal aneurysmal bone cysts. J Pediatr Orthop. 2016;36(2):205–212. [DOI] [PubMed] [Google Scholar]
- 51. Bakri MH, Ismail EA, Ali MS, Elsedfy GO, Sayed TA, Ibrahim A. Behavioral and emotional effects of repeated general anesthesia in young children. Saudi J Anaesth. 2015;9(2):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]