Figure 1. Impact of the tubulin economy on the microtubule cytoskeleton.
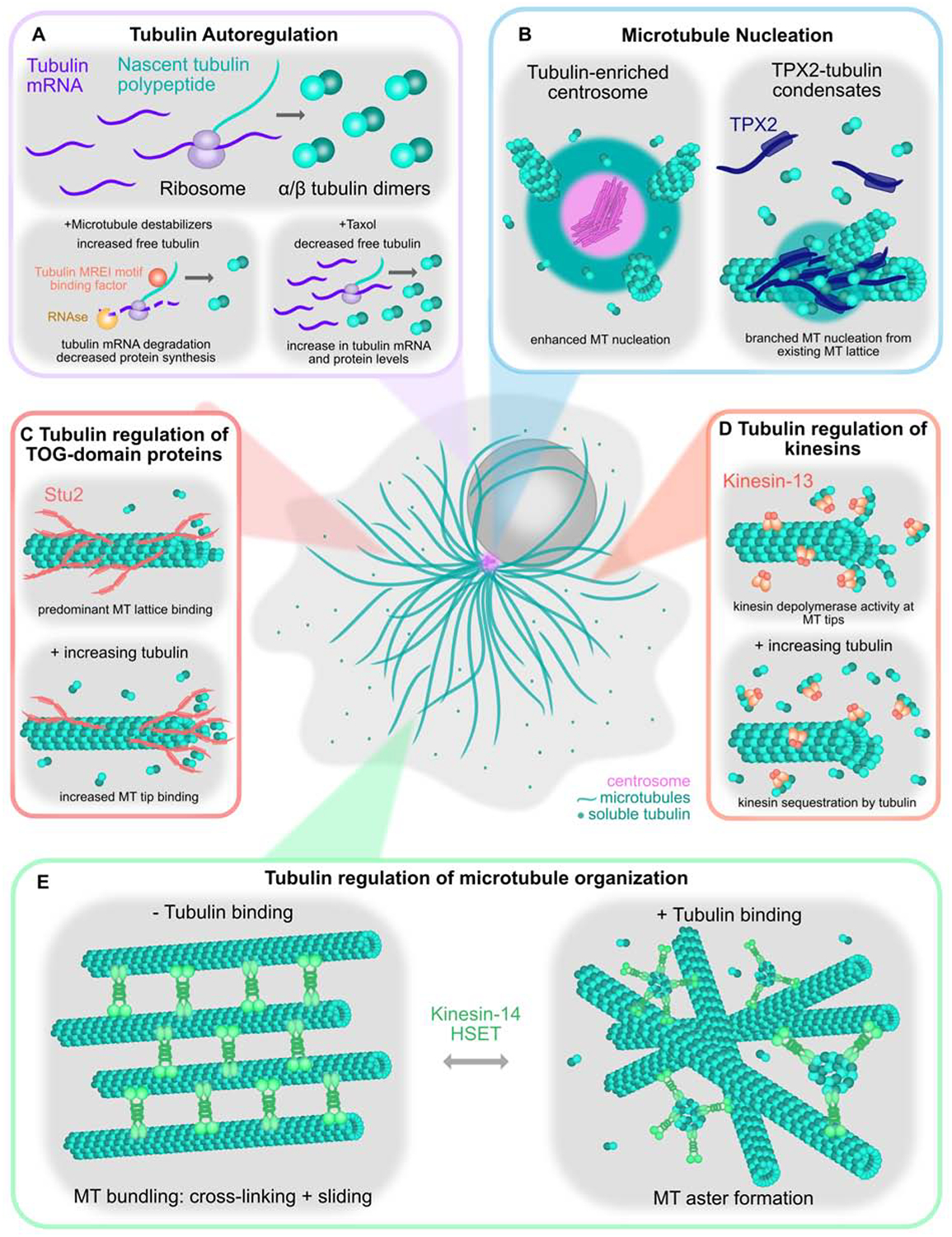
A) Soluble (unpolymerized) tubulin controls the stability of tubulin mRNAs, and therefore controls the cellular levels of tubulin proteins. Microtubule destabilizing agents cause an increase in the available pool of soluble tubulin, which triggers degradation of tubulin mRNA, resulting in a decrease in tubulin protein synthesis. Tubulin autoregulation occurs by recognition of a ribosome-associated nascent tubulin peptide by TTC5, which leads to RNAse digestion of tubulin mRNA transcripts. Conversely, treatment of cells by taxol decreases the available pool of soluble tubulin and causes an increase in tubulin mRNA levels and increased tubulin protein synthesis. B) Pockets of highly concentrated tubulin within the cell create sites of enhanced microtubule nucleation. The centrosome, for example, concentrates tubulin ~10-fold. TPX2 forms co-condensates with tubulin, leading to enhanced microtubule nucleation both in solution, and along existing microtubule polymers. C) Tubulin can regulate microtubule dynamics indirectly through regulation of microtubule-associated proteins. Soluble tubulin affects the localization of TOG-domain protein Stu2; in the absence of tubulin, Stu2 has a higher affinity for the microtubule lattice, however, Stu2 shifts to the microtubule tip in the presence of tubulin, where it acts as a microtubule polymerase. D) Kinesin-13 family motors promote the dissociation of terminal tubulin dimers, which increases the frequency of microtubule catastrophes. A by-product of this reaction is the formation of a tubulin-motor complex, which occurs following dissociation of the motor from the microtubule end. In principle, kinesin-13 motors may therefore be subject to product-inhibition, ultimately decreasing the depolymerase activity of the enzyme. E) Soluble tubulin controls the ability of kinesin-14 HSET to organize microtubules into different types of architectural arrays. HSET as a single molecule may bind across multiple microtubules through both its motor and tail domains, therefore promoting microtubule bundling. HSET will crosslink parallel bundles, but slide antiparallel bundles through its minus-end directed motility. Binding of soluble tubulin enables HSET form multi-motor clusters, which are then capable of organizing microtubules into aster-like formations.
