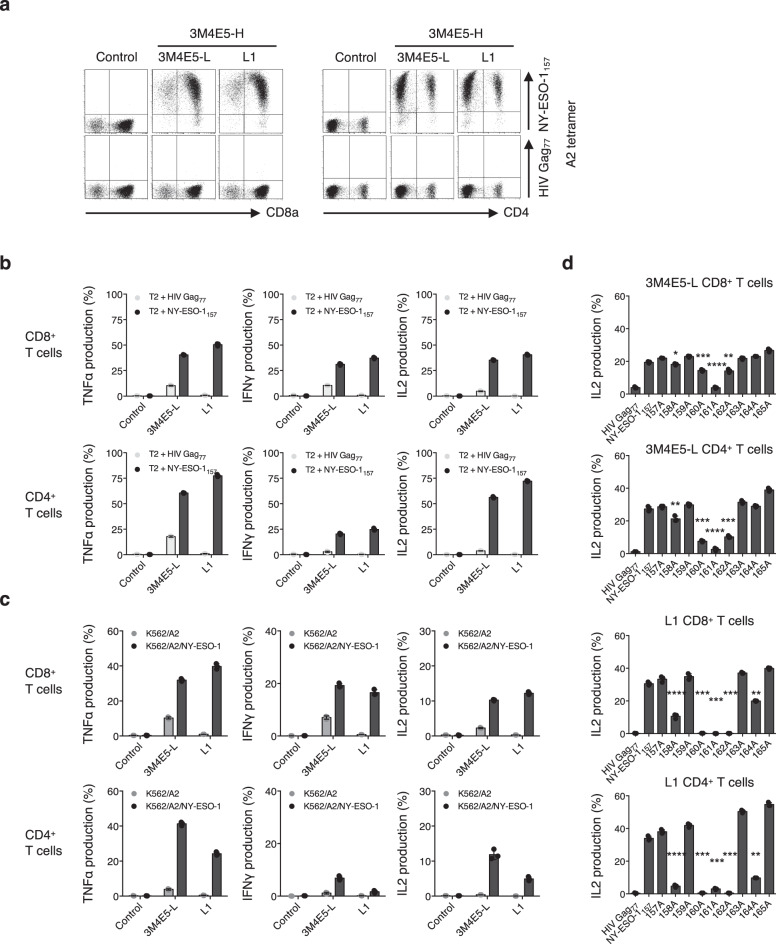
Fig. 3. Sufficient antitumor reactivity and minimized cross-reactivity of fine-tuned scFv-expressing A2/NY-ESO-1157 CAR-T cells.
a Clone L1, or the original 3M4E5-L second generation (CD28ζ) CAR was transduced into peripheral blood T cells. Control T cells and these transfectants were stained with 20 μg/mL A2/NY-ESO-1157 tetramer or A2/HIV Gag77 tetramer. Representative dot plots of both CD8+ T cells and CD4+ T cells are shown. b, c CAR-T cells generated as above were incubated with the indicated peptide-pulsed T2 cells (b), K562/A2 cells, or K562/A2/NY-ESO-1 cells (c), and their cytokine production was measured by intracellular cytokine assays. The experiments were performed in triplicate, and error bars depict the SD. d L1 CAR or original 3M4E5-L CAR CD8+ T cells and CD4+ T cells were incubated with T2 cells pulsed with 9 different alanine-substituted peptides at 10 μg/mL. IL2 production (%) was measured by intracellular cytokine assays. Each response to alanine-substituted peptides was compared with the response to the original NY-ESO-1157 peptide. The experiments were conducted in triplicate, and error bars depict the SD. Welch’s t test (two-sided) was performed for comparison. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Note that control T cells were generated by transduction of the ΔNGFR gene into human peripheral blood T cells. Gene-modified T cells with similar transduction efficiency were prepared, and then ΔNGFR-positive cells were gated and analyzed (a–d).