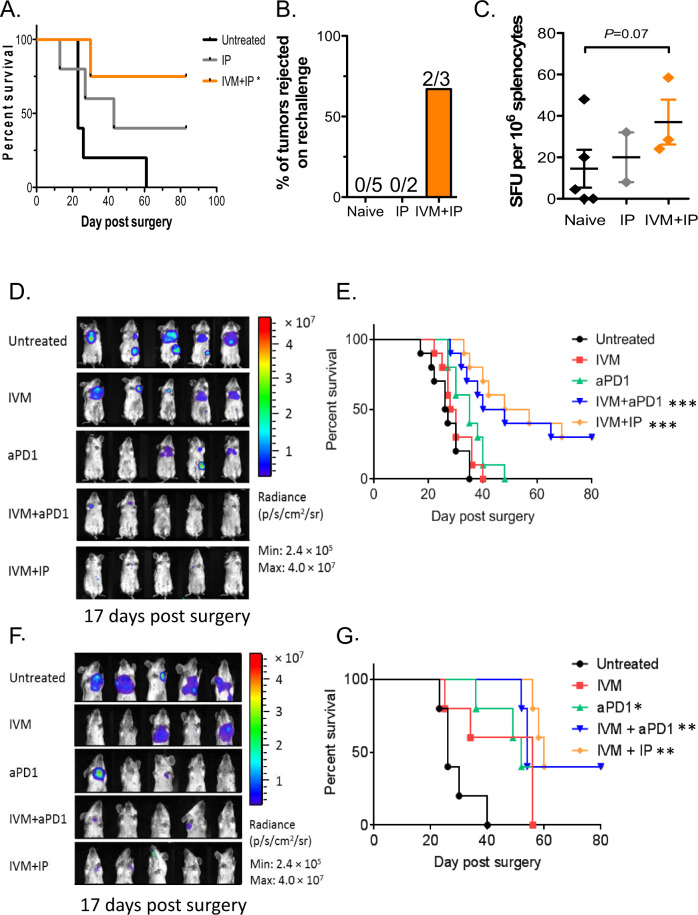
Fig. 4. Combination ivermectin and IP therapy in the neoadjuvant, adjuvant, and metastatic settings.
A Survival of animals following surgical resection of primary tumor (on day 16 post tumor inoculation). B Induction of protective immunity in treated mice that survived beyond day 80, then re-challenged with 4T1 cells on the contralateral mammary fat pad. C IFNγ ELISPOT analysis of 4T1-reactive splenocytes in treated animals. Mean ± s.d., n = 5 mice, pooled data from two independent experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. D In vivo bioluminescence imaging of mice (on day 17 post surgery and after completion of the entire treatment schedule) treated with ivermectin, anti-PD1, ivermectin + anti-PD1 ± IL-2 (IP), or control in the adjuvant setting. Mean ± s.d., n = 5 mice, pooled data from two independent experiments. E Survival of animals in the adjuvant setting following surgical resection of primary tumor burden and treated starting 2 days after with ivermectin, anti-PD1, ivermectin + anti-PD1 ± IL-2 (IP), or control; n = 5 mice per group, two-tailed log-rank test; **p ≤ 0.01, ****p ≤ 0.0001. F In vivo bioluminescence imaging (on day 17 post surgery and after completion of the entire treatment schedule) of mice with documented metastasis, then treated with ivermectin, anti-PD1, ivermectin + anti-PD1 ± IL-2 (IP), or control. Mean ± s.d., n = 5 mice, pooled data from two independent experiments. G Kaplan–Meier survival analysis of mice in the metastatic setting treated with ivermectin, anti-PD1, ivermectin + anti-PD1 ± IL-2 (IP), or control; n = 5 mice per group, two-tailed log-rank test; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.