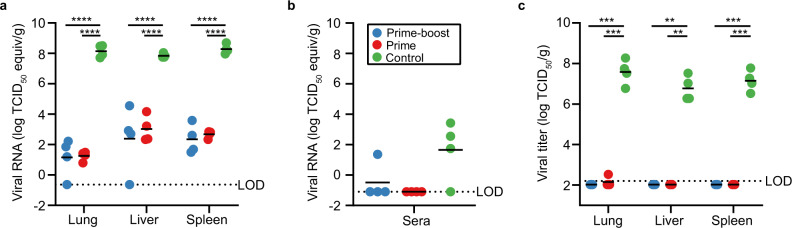
Fig. 5. Viral load is significantly lower in the tissues and sera of ChAdOx1-Lassa-GPC vaccinates compared to controls.
Lung, liver, spleen, and sera samples were collected from animals (n = 4/group) during post-challenge necropsy (D12). Detection of LASV RNA in the tissues (a) and sera (b) of vaccinated and control animals by qRT-PCR. The dotted lines represent the limits of detection (LOD) for analysis of tissue (−0.64 log TCID50/g equivalents) and sera (−1.10 log TCID50/mL equivalents) due to distinct inactivation methods used to remove these samples from BSL4 containment. Differences in viral load in the lung, liver, and spleen of vaccinated animals versus controls were statistically significant by two-way ANOVA with Tukey’s multiple comparisons test (****p < 0.0001). Levels of LASV RNA detected in sera did not differ significantly between groups by Kruskal–Wallis nonparametric test with Dunn’s multiple comparisons. c Infectious virus titers in tissues positive for LASV RNA (lung, liver, and spleen) were determined via endpoint titration on Vero E6 cells. The dotted line represents the LOD (2.20 log TCID50/g). Differences in virus titers between vaccinated and control animals were statistically significant in all tissues by two-way ANOVA with Tukey’s multiple comparisons test (**p = 0.0012, ***p = 0.0008, 0.0006, 0.0002).