Figure 2. MavQ is a phosphoinositide 3 Kinase that converts PtdIns into PtdIns3P.
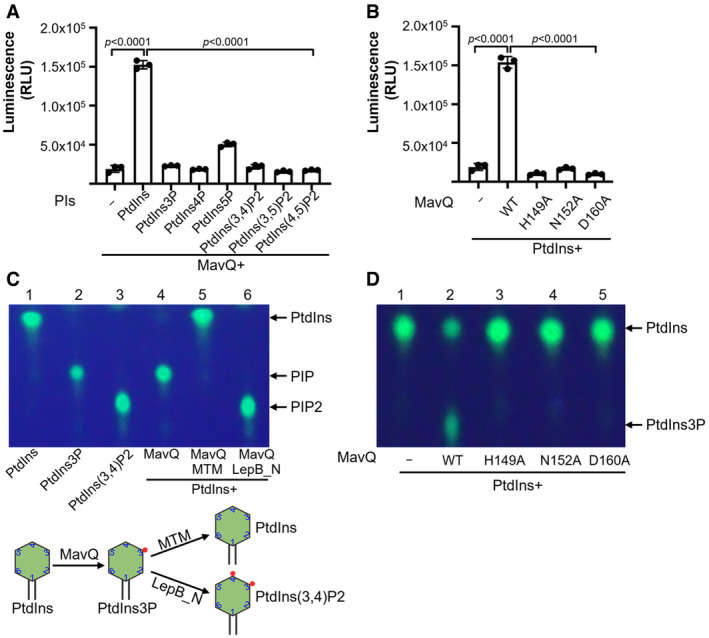
- Biochemical assays for the kinase activity of MavQ. Purified MavQ was incubated with or without specific PI substrates, and the conversion of ATP to ADP was measured by the ADP‐Glo assay. RLU, relative luminescence units.
- H149, N152, and D160 in MavQ are critical for its kinase activity. Purified wild‐type and mutant MavQ were reacted with PtdIns and ATP. The kinase activity was determined by the ADP‐Glo assay.
- The PI product produced by MavQ determined by TLC assays. di‐C8‐Bodipy‐FL‐PtdIns was used to react with MavQ (lane 4), and samples were further incubated with the 3‐phosphatase MTM which hydrolyzes both PtdIns3P and PtdIns(3,5)P2 (lane 5) or with the PI4K LepB_NTD which phosphorylates PtdIns3P into PtdIns (3,4)P2 (lane 6). A schematic illustration of the enzymatic reactions is shown in the lower panel.
- Mutations in H149, N152, and D160 abolish the ability of MavQ to convert PtdIns into PtdIns3P. Experiments were performed with the same procedure as described in panel C.
Data information: In (A and B), data are shown as mean ± SD of triplicates (technical replicates, n = 3) and are representative of three independent experiments (biological replicates, n = 3). In (C and D), data are representative of at least three independent experiments (biological replicates, n ≥ 3). Unpaired two‐tailed Student’s t‐test, p < 0.05 indicates significant difference.
Source data are available online for this figure.
