Figure 4.
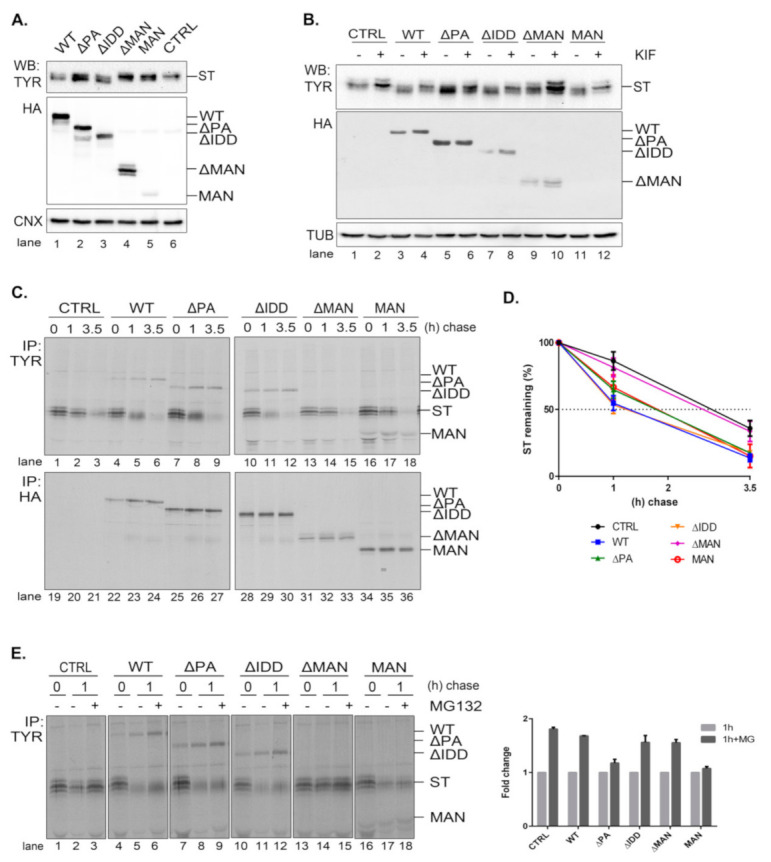
Degradation of soluble tyrosinase in the presence of EDEM3 mutants. (A) Soluble tyrosinase (ST) is processed differently by EDEM3 constructs. EDEM3-KO cells were co-transfected with plasmids encoding for soluble tyrosinase (ST) and EDEM3 mutants and processed for Western blotting with polyclonal anti-tyrosinase (TYR) antibodies and monoclonal anti-HA. Calnexin (CNX) was used as loading control. (B) Kifunensine impairs mannosidase properties of EDEM3 constructs. Same as in (A) except cells were treated with 25 µM kifunensine for 16h prior to harvesting. Tubulin (TUB) was used as loading control. (C) ST degradation rate is modulated by EDEM3 mutant proteins. EDEM3-KO cells were co-transfected with plasmids encoding for ST and EDEM3 constructs and subjected to pulse-chase analysis. Half of cell lysate was incubated ON with HM anti-tyrosinase (TYR) antibodies (upper panel) while the other half was incubated with mouse anti-HA antibody (lower panel). The resulting immunocomplexes were separated by SDS-PAGE. Bands were visualized by autoradiography. (D) Graphic representation of ST degradation rate from (C) as (mean ± SD) of three independent experiments. (E) Proteasomal degradation of ST is fine-tuned by EDEM3 constructs. Same as in ((C), upper panel), except cells were treated with 10 µM MG132 for 1 h prior to immunolabeling. Band densitometry quantification of treated condition versus non-treated at 1 h chase is presented as the mean ± SEM of two independent experiments.
