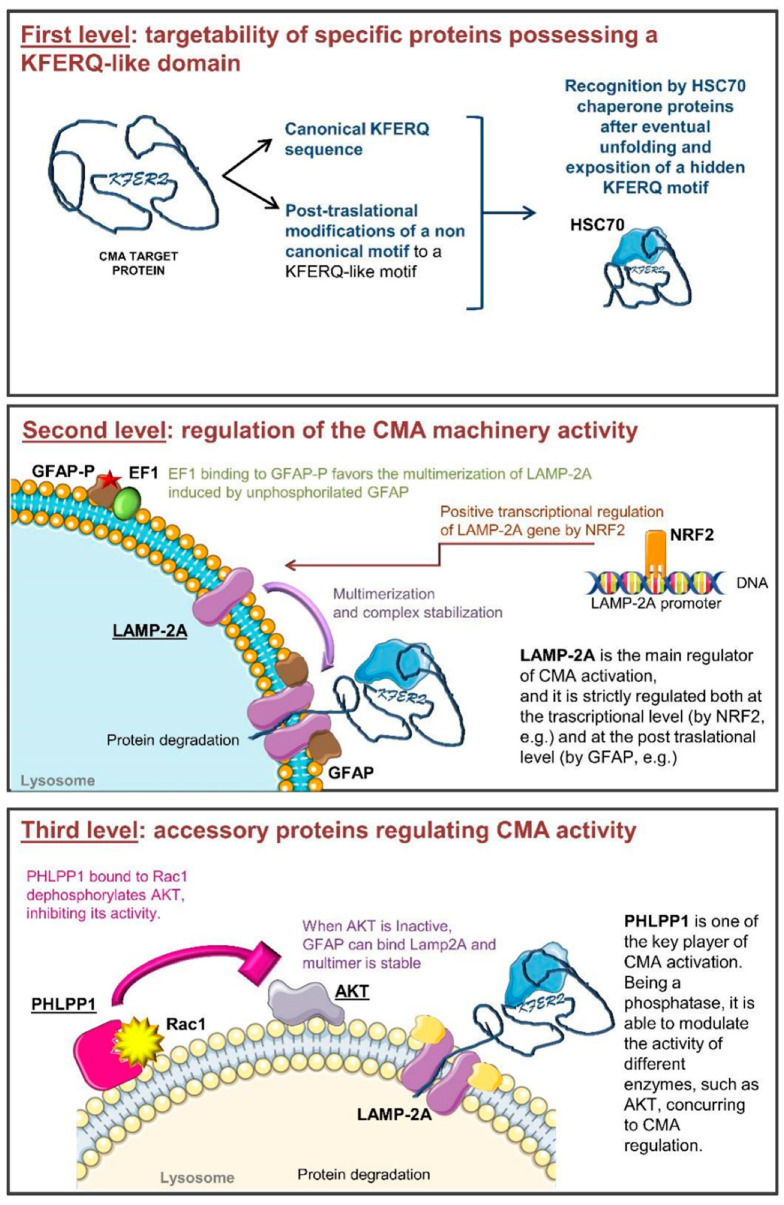
Figure 1.
Schematic representation of the multi-level CMA (chaperone-mediated autophagy) regulation machinery. Panel 1: The first regulatory level is based on the availability and accessibility of the KFERQ motif. Non-canonical motifs can be modified to be recognized by chaperone proteins. HSC70 chaperone delivers specific proteins to the lysosome. Panel 2: The second regulatory level depends on LAMP-2A quaternary structure. Its trafficking in the lysosomal membrane is finely regulated. Moreover, unphosphorylated GFAP (GFAP) stabilizes the LAMP-2A multimer, favoring the translocation of unfolded proteins within the lysosomes. When GFAP is present in its phosphorylated form (GFAP-P), it interacts with EF-1α, determining the disassembling of LAMP-2A multimers. In addition, LAMP-2A de novo expression can be regulated by the transcription factor NRF-2. Panel 3: The third regulatory level is due to the availability of accessory proteins among which the main regulator is PHLPP1. It is a phosphatase influencing the activity of other proteins. Rac-1 bound to PHLPP1 favors its activity on AKT. Inactive AKT is not able to phosphorylate GFAP allowing LAMP-2A multimer stabilization.