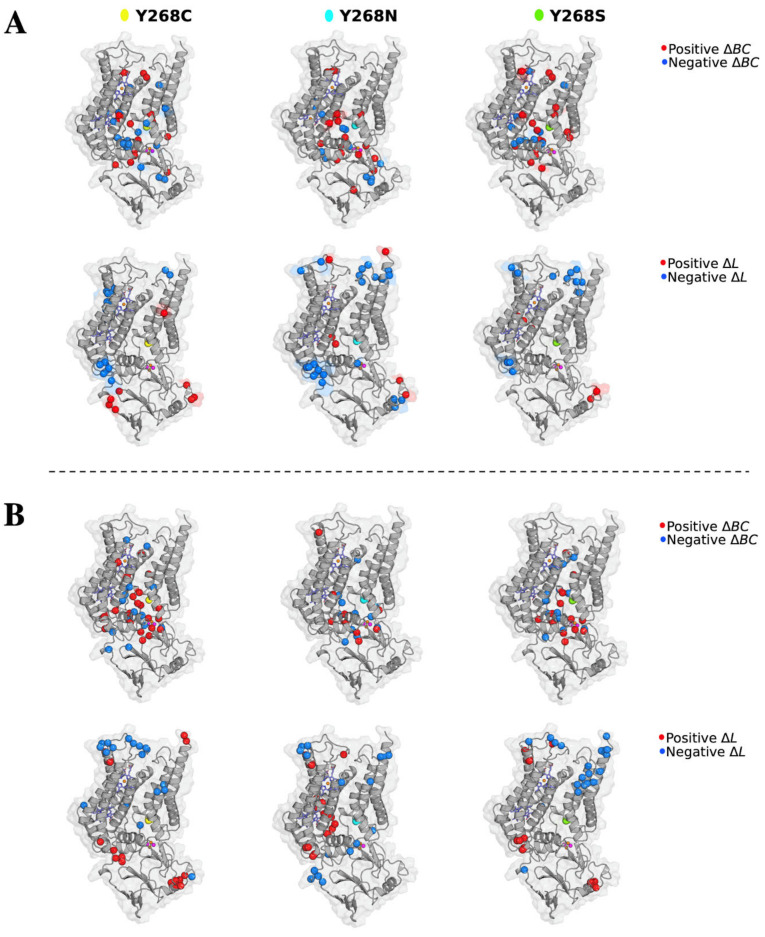
Figure 10.
Effect of the three mutations Y268C, Y268N and Y268S on the protein intra-communication in the holo protein. (A) PfCytb-ISP holo protein (B) PfCytb-ISP ATQ-bound protein. Residues exhibiting a significant change in average betweenness centrality (ΔBC) are mapped onto the structure. The change in ΔBC was calculated as WT (wild-type) — mutant. The red spheres therefore represent residues with positive ΔBC values, i.e., residues that lost centrality in the mutant model relative to the WT. The blue spheres represent residues with negative ΔBC values, i.e., residues that gained more centrality in the mutant models relative to the WT (B) Residues exhibiting a significant change in average accessibility (ΔL) are mapped onto the structure. Change in ΔL was calculated as WT — mutant. The red spheres therefore represent residues with positive ΔL, i.e., residues that increased accessibility in the mutant model relative to the WT. The blue spheres represent residues with negative ΔL values, i.e., residues that decreased accessibility in the mutant model relative to the WT.