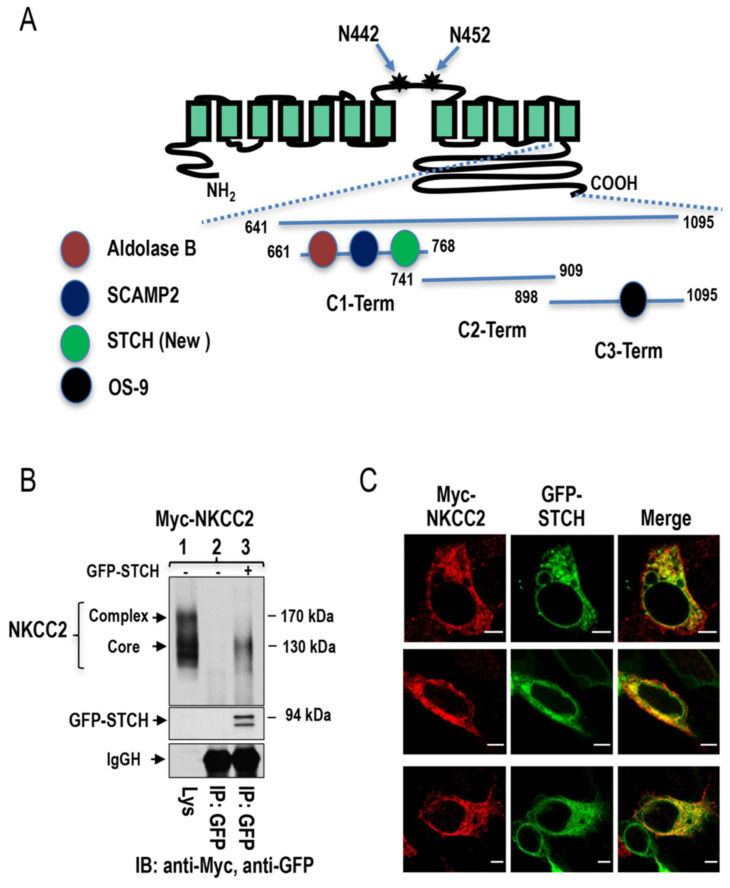
Figure 1.
Identification of STCH as a novel NKCC2-interacting protein. (A) Mouse NKCC2 yeast two-hybrid baits constructs. A proposed topology for sodium-coupled chloride co-transporter NKCC2. N442 and N452 are the potential N-glycosylation sites. As previously described, mouse NKCC2 C terminus was divided into three peptide fragments (C1-term, C2-term, and C3-term) used as baits for the yeast two-hybrid. Similar to Aldolase B [39] and SCAMP2 [40], STCH interacts with C1-term while OS9 binds to C3-term [28]. (B) NKCC2 binds, in vivo, to STCH in HEK cells. HEK cells transiently transfected with Myc-NKCC2 singly or in combination with GFP-STCH were immunoprecipitated (IP) with anti-GFP anti-body (lanes 2 and 3). 5% of total cell lysate (Lys) was resolved as positive control. Co-immunoprecipitated NKCC2 and STCH proteins were detected by immunoblotting (IB) using anti-Myc (lane 3) and anti-GFP respectively (lane 3). IgGH, the heavy chain of IgG. The positions of immature (core glycosylated) and mature (complex-glycosylated) proteins of NKCC2 are indicated. The interaction of NKCC2 with STCH involves mainly the immature form of the co-transporter. (C) Imunofluorescence confocal microscopy showing distribution of Myc-NKCC2 and GFP-STCH in HEK cells. Fixed and permeabilized cells were stained with mouse anti-Myc for NKCC2 (Texas Red). The yellow color (merged image) indicates co-localization of the proteins. Bars, 5 μm.