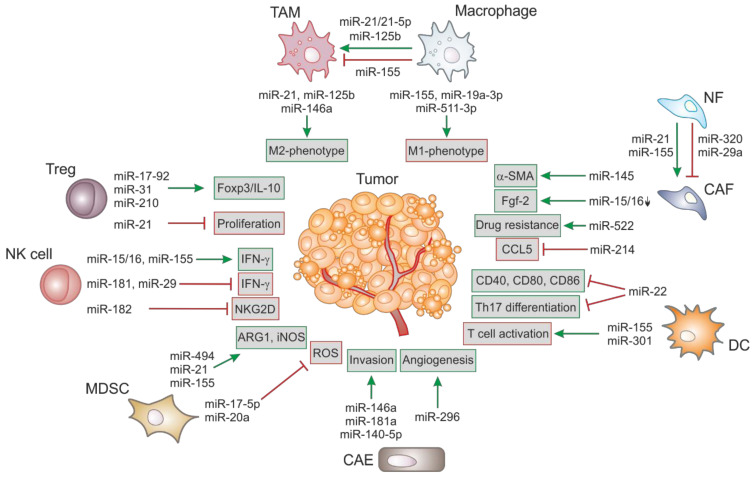
Figure 1.
Examples of dysregulated microRNAs (miRs) in cells of the tumor microenvironment (TME) and their impact on tumor cells. MiRs can either act as tumor-suppressors by regulating molecules or pathways with anti-tumoral characteristics (red box; red T bar) or oncomiRs that directly or indirectly impact on tumor-promoting genes and protein networks (green box; green arrow). The differential expression of miRs in macrophages and tumor-associated macrophages (TAMs), or the uptake of exogenous miRs, modulate their polarization. Similarly, miRs expressed in cancer-associated fibroblasts (CAFs) regulate their migration, cytokine production, and trans-differentiation as well as tumor growth. CAF-derived miRs (e.g., miR-522) can also enhance drug resistance of tumor cells. In dendritic cells (DCs), miRs regulate Th17 differentiation, co-stimulatory molecule expression, and T cell activation. miRs expressed in cancer-associated endothelial cells (CAEs) regulate the microvascular invasion and angiogenesis activity to drive tumorigenesis. In myeloid-derived suppressor cell (MDSCs), miRs modulate the expansion/immune-suppressive functions. In natural killer (NK) cells, miRs modulate the production of effector molecules (e.g., IFN-γ) and the activating receptor encoded by killer cell lectin like receptor K1 (NKG2D). Furthermore, miRs regulate the expression of transcription factors and cytokine production of regulatory T cells (Tregs). ARG1, arginase 1; α-SMA, α-smooth muscle actin; CAE, cancer-associated endothelial cell; CAF, cancer-associated fibroblast; DC, dendritic cell; Fgf2, fibroblast growth factor 2; IFN, interferon; iNOS, inducible NO synthase; NF, normal fibroblast; NKG2D, encoded by killer cell lectin like receptor K1; ROS, reactive oxygen species; TAM, tumor-associated macrophage; Treg, regulatory T cell.