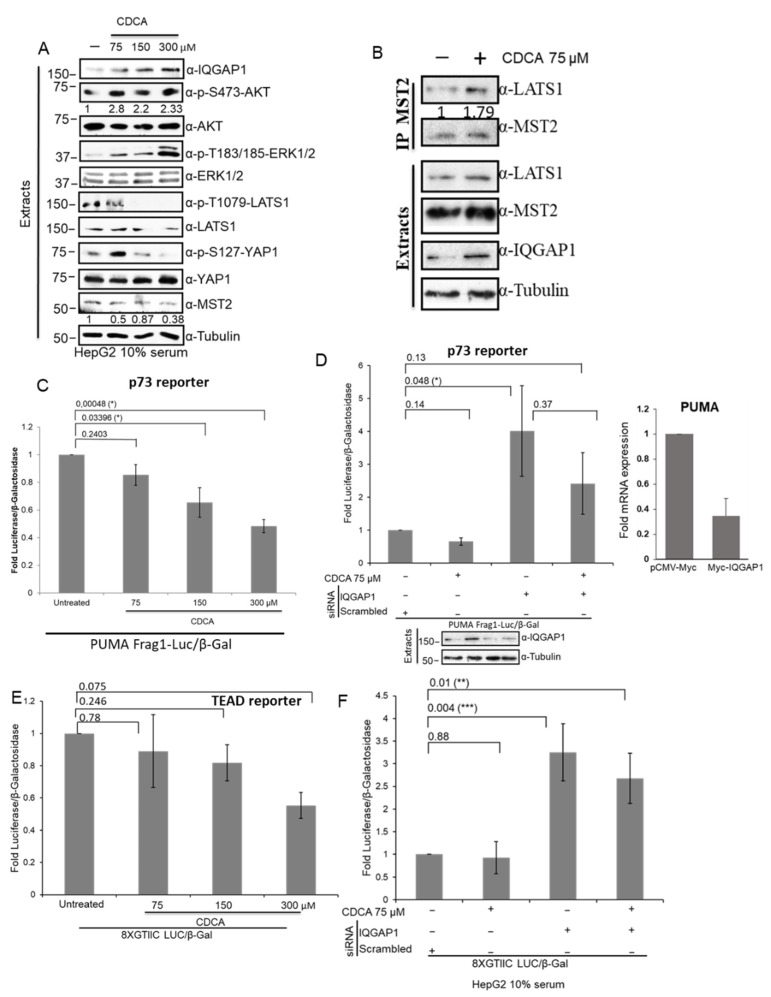
Figure 7.
CDCA overload induces IQGAP1 expression and deactivates Hippo signalling. (A) HepG2 cells were treated with increasing concentrations of CDCA (75, 150 or 300 µM) for 8 h and the cells were lysed. Cell extracts were analysed by western blotting with indicated antibodies. Blots were quantified using ImageJ and the numbers show relative fold change of AKT phosphorylation normalised by total levels of AKT and level of expression of MST2 normalised by tubulin levels. (B) HepG2 cells were treated with 75 µM CDCA for 8 h. MST2 was immunoprecipitated from cell extracts using specific antibody. Immunoprecipitated proteins and cell extracts were blotted with the indicated antibodies. IP blots were quantified using ImageJ and the numbers show relative fold change of LATS1 normalised by MST2 blot. (C) HepG2 cells were co-transfected with PUMA promotor luciferase reporter (PUMA Frag1-Luc, p73 reporter) and β-galactosidase construct. Forty-eight hours after transfection, the cells were treated for 8 h with the indicated concentrations of CDCA. p73 transcriptional activity was measured by luminescence and β-galactosidase enzymatic activity was measured by absorbance. (D) Left panel. HepG2 cells were co-transfected with PUMA and β-galactosidase reporters and 50 ng/mL IQGAP1 or non- targeting siRNA pool where indicated. Forty-eight hours after transfection, the cell were treated for 8 h with 75 μM CDCA. Transcriptional activity was measured as in (C). Lower panel shows protein expression of the indicated proteins determined by western blot in a representative experiment. Right panel. HepG2 cell were transfected with 0.5 μg Myc-IQGAP1 or pCMV-Myc plasmids and PUMA mRNA expression was measured by rtPCR. The graph shows PUMA mRNA levels normalised by GAPDH mRNA expression. (E) HepG2 cells were transfected with 8XGTIIC LUC construct (TEAD reporter) β-galactosidase plasmid. Forty-eight hours after transfection, the cells were treated for 8 h with increasing concentrations of CDCA. TEAD transcriptional activity was measured and β-galactosidase enzymatic activity were measured as in C. (F) HepG2 cells were co-transfected with 8XGTIIC LUC construct (TEAD reporter) β-galactosidase plasmid and 50 ng/mL IQGAP1 or non-targeting siRNA pool, where indicated. Forty-eight hours after transfection, the cells were treated for 8 h with 75 μM CDCA. After lysis, TEAD transcriptional activity was determined as in (C). p-values were obtained by Student’s t-test, n = 3, error bars indicate mean SEM. * = p < 0.05, ** = p < 0.01, *** = p < 0.001.