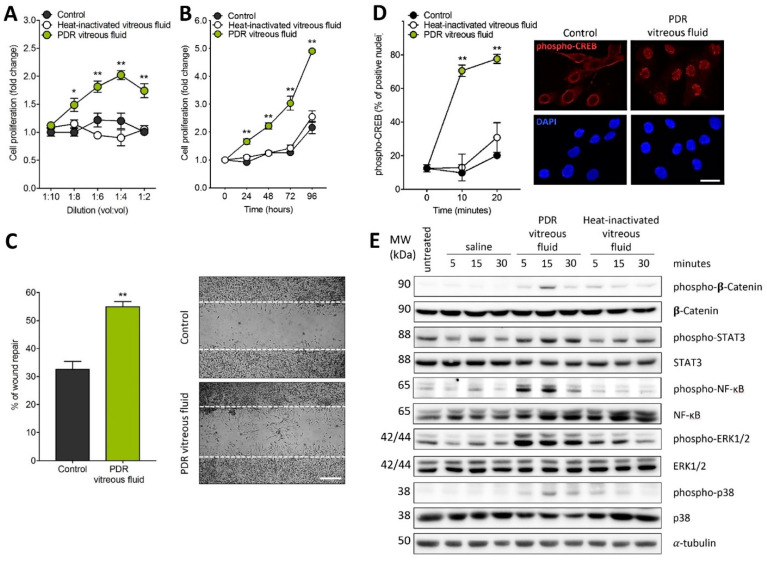
Figure 1.
Müller cell activation by proliferative diabetic retinopathy (PDR) vitreous. (A,B) MIO-M1 cells were treated with increasing amounts of PDR or heat-inactivated vitreous samples (vol:vol dilution in cell culture medium) and counted 72 h thereafter (A) or were incubated with 1:4 vitreous dilution and counted at different time points (B). Cell proliferation was expressed as fold change in respect to the control. Data are the mean ± SD of 5 independent experiments. * p < 0.05 and ** p < 0.01 vs. control or heat-inactivated vitreous fluid, one-way ANOVA. (C) Wounded MIO-M1 monolayers were treated with PDR vitreous fluid. After 24 h, MIO-M1 cells invading the wounded area were quantified by computerized analysis of the digitalized images. Data are the mean ± SD of 2 independent experiments (8 microscopic fields per experimental point). ** p < 0.01 vs. control, Student’s t test. Inset: representative images of the repaired area in control cells (upper panel) and PDR vitreous fluid-treated cells (lower panel). Scale bar = 500 µm. (D) MIO-M1 cells were treated with PDR or heat-inactivated vitreous samples. After 0–20 min, the percentage of phospho-CREB immunoreactive MIO-M1 nuclei were quantified (n = 80 cells per experimental point). ** p < 0.01 vs. control or heat-inactivated vitreous, one-way ANOVA. Inset: phospho-CREB immunoreactivity (red) in MIO-M1 cells at 10 min in control (left panels) and after PDR vitreous treatment (right panels); nuclei were stained with DAPI (blue). Data are representative of 2 independent experiments. Scale bar = 25 µm. (E) Western blot analysis of the phosphorylation of the signaling proteins β-catenin, STAT3, NF-κB, ERK1/2, and p38 in MIO-M1 cells following 0–30 min of stimulation with PDR or heat-inactivated vitreous samples. Data are representative of 2 independent experiments that gave similar results. MW, molecular weight. Densitometric analysis of the Western blot membranes is shown in Figure S2.