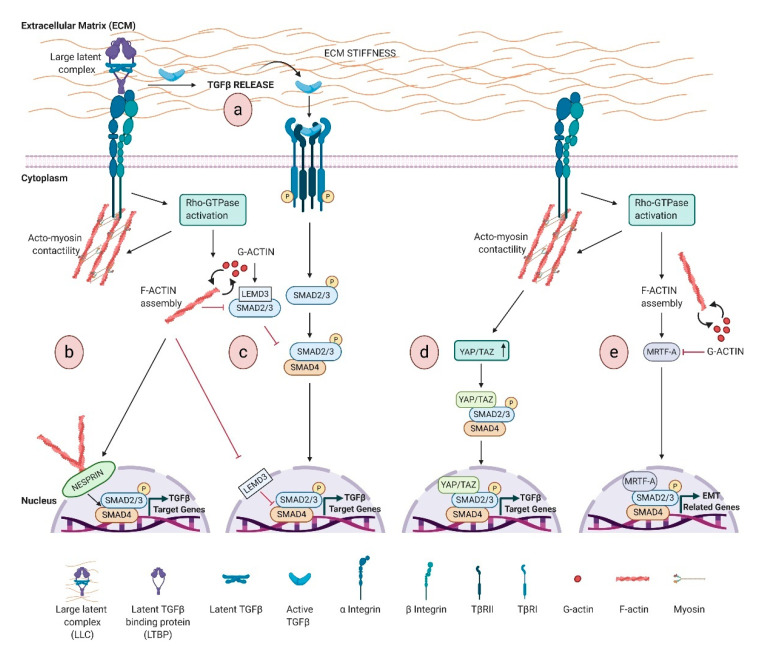
Figure 1.
Schematic representation of transforming growth factor β (TGFβ) signaling regulation by actin remodeling induced by extracellular matrix (ECM) stiffness. Activation of Rho GTPase by mechanical cues promotes F-actin assembly and actin cytoskeleton contractility, which activates TGFβ signaling by: (a) Inducing TGFβ ligand release and activation. The ECM-integrin-actin cytoskeleton linkages allow integrin to shift toward an active configuration that favors TGFβ release from LTBP/latent TGFβ complex. Active TGFβ initiates signaling via TGFβ receptors, which ultimately drives the phosphorylation-dependent formation of SMAD2/3-4 complex. This complex translocates to the nucleus and facilitates the transcription of TGFβ-dependent genes; (b) Accumulating at the front of the nucleus of the lamin-binding protein Nesprin-2, which induces SMAD nuclear localization; (c) Inhibiting the formation of both cytosolic and nuclear LEMD3-SMAD2/3 complexes, resulting in the relief of LEMD3 negative regulation; (d) Activating YAP/TAZ pathway, which regulates both SMAD2/3 shuttling and SMAD-dependent transcriptional activity; (e) Controlling myocardin-related transcription factor A (MRTF-A) localization to mediate the SMAD-dependent transcriptional activity.