Abstract
Background: Systemic photoprotection (i.e., administration of substances such as nicotinamide, carotenoids, and vitamin D) may be important to reduce photocarcinogenesis or to support long-term protection against UV irradiation. Clinical trials showed that oral nicotinamide is effective in reducing the onset of new nonmelanoma skin cancers (NMSCs), while other oral photoprotectors failed to achieve the reduction of new melanoma or NMSC formation in humans. The aim of this study was to summarize the current state of knowledge of systemic photoprotection and to evaluate the knowledge and attitude of dermatologists regarding these treatments. Methods: The survey was conducted on a sample of dermatologists recruited according to a snowball sampling procedure. The questionnaire consisted of a first part asking for characteristics of the participant and a second part with 12 specific questions on their knowledge about systemic photoprotection, particularly their knowledge of astaxanthin, β-carotene, nicotinamide, and vitamin D3. Results: One hundred eight dermatologists answered the survey. Most of them (85.2%) stated that oral photoprotectors have a role in the prevention of skin cancer, and responses mainly mentioned nicotinamide. More than half of them (54.6%) had prescribed all the considered oral photoprotectors, but the majority of them had prescribed nicotinamide, mainly for 2 to 3 months during summer, almost invariably (n = 106) associated with topical photoprotectors. Most dermatologists (>80%) were aware of scientific publications demonstrating an effect of systemic photoprotectors on NMSC. Conclusions: Most Italian dermatologists have positive views on oral photoprotection in skin cancer and are aware of the demonstrated potential of nicotinamide in the prevention of NMSCs.
Keywords: photoprotection, sunscreen, skin tumor, skin cancer, melanoma, nicotinamide, dermatology
1. Introduction
Topical and systemic photoprotection approaches are the first-line prevention strategies for skin cancers, including melanoma and nonmelanoma skin cancers (NMSCs). Topical photoprotection may present limitations owing to the inadequate application or the especially short half-life on the skin, which requires frequent reapplication and risks potential side effects [1,2,3]. On the other hand, oral photoprotectors do not directly protect the skin against the damage induced by UV irradiation and may cause potential side effects, and internal factors may modify some oral photoprotector molecules. However, oral photoprotectors have some advantages, such as the ease of use, the efficiency that is not modified by external factors, and the possibility to estimate the half-lives of the different molecules [3]. Systemic photoprotection, consisting in the administration of substances such as nicotinamide, carotenoids, polyphenols, and other antioxidants, is important for reducing photocarcinogenesis or to support long-term protection against UV irradiation [3,4,5,6,7].
Notably, it has been demonstrated in clinical trials that the oral consumption of nicotinamide is effective in reducing the onset of new NMSCs [6,7,8]. Carotenoids can suppress in vitro and in vivo the formation of UVA- and UVB-mediated reactive oxygen species, preventing the photoinactivation of antioxidant enzymes and the induction of DNA damage. A randomized controlled trial studied β-carotene supplementation in the prevention of NMSC, reporting that there was no beneficial or harmful effect on the rates of new skin cancers [9]. So far, the efficacy of carotenoids in reducing new skin cancers in humans has not been demonstrated [5,9,10]. The active derivative of vitamin D (1,25(OH)2 D3) enhances the survival of skin cells following exposure to UV radiation by reducing the level of damage to DNA and thus reducing UV-induced apoptosis in preclinical studies [11]. Furthermore, 1,25(OH)2 D3 has been shown to reduce postirradiation edema, inflammation, and photocarcinogenesis in mouse skin [11]. In a recent systematic review and dose–response meta-analysis of prospective studies regarding vitamin D intake and skin cancer risk [12], the authors reported that, while intakes of dietary or supplemental vitamin D were not associated with the risk of melanoma and squamous cell carcinoma, high intakes of vitamin D from diet and supplements were slightly associated with basal cell carcinoma risk. Considering that vitamin D levels are mainly affected by exposure to the sun, a higher risk of skin cancer may be confounded by sun exposure [12]. Other antioxidants, such as astaxanthin and other molecules, fail to demonstrate the capacity to reduce new melanoma or NMSC formation [1,3].
Considering the increasing incidence of both melanoma and NMSC worldwide and the important public health issues posed by these tumors, topical and systemic photoprotection are vital to reduce the occurrence of such tumors. In fact, several factors such as the continuous increase in life expectancy in the general population, higher phototypes, and lower latitudes favor the onset of these cancers; however, the most important factor is the cumulative exposure to UV radiation.
The aim of this study was to summarize the current state of knowledge of systemic photoprotection and to evaluate the knowledge and attitude of dermatologists regarding these treatments.
2. Materials and Methods
The survey was conducted on a sample of dermatologists recruited by other dermatologists from among their acquaintances according to a snowball sampling procedure. The study was approved by the Institutional Ethical Committee (Approval # 608-1) of IDI-IRCCS in Rome, Italy, and was conducted in accordance with the Declaration of Helsinki. A questionnaire was sent by email to all clinicians, describing the purpose of the study. Those who agreed to participate signed a written informed consent before entering the study. Data were collected in June 2020.
The questionnaire consisted of a first part asking for characteristics of the participant, i.e., gender, number of years since they finished dermatology training (<10, 10–19, ≥20), geographical area (Northern, Central, or Southern Italy), workplace (hospital, university or research hospital, local health department, private practice). Regions included in each geographical area were as follows: in Northern Italy, Valle d’Aosta, Piemonte, Lombardia, Liguria, Veneto, Trentino Alto Adige, Friuli Venezia Giulia, and Emilia Romagna; in Central Italy, Toscana, Lazio, Umbria, and Marche; and in Southern Italy, Abruzzo, Molise, Campania, Basilicata, Puglia, Calabria, Sicilia, and Sardegna. In the second part, clinicians were asked to answer 12 specific questions about systemic photoprotection. The systemic photoprotectors which were listed in the questions were astaxanthin, β-carotene, nicotinamide, and vitamin D3.
Data were described as numbers and percentages. Results were compared in different subgroups of participants, according to gender, years since finishing dermatology training, geographical area, and workplace, using the chi-square test. Data were analyzed using IBM SPSS Statistics for Windows, Release 26.0.0.1 (IBM Corp., Armonk, NY, USA).
Two multivariable logistic models were tested using Question 2 (“Do you believe that oral photoprotectors may have a role in the prevention of skin cancers?”) and Question 7 (“Are you aware of scientific studies/trials that have demonstrated the reduction of NMSC due to one of the products indicated in systemic photoprotection?”) as dependent variables, respectively. Independent variables were gender, years since finishing dermatology training, geographical area, and workplace.
Furthermore, we reported the grade of recommendation and level of evidence regarding the efficacy of the mentioned oral photoprotectors based on clinical and preclinical studies from scientific literature in reducing the incidence of NMSC. We considered the grade of recommendation (from A to D) and the level of evidence (1a, 1b, 2a, 2b, 3a, 3b, 4, 5) based on the Centre for Evidence-Based Medicine.
3. Results
Knowledge of Oral Photoprotection among Dermatologists and Grade of Recommendation or Level of Evidence of Oral Photoprotectors
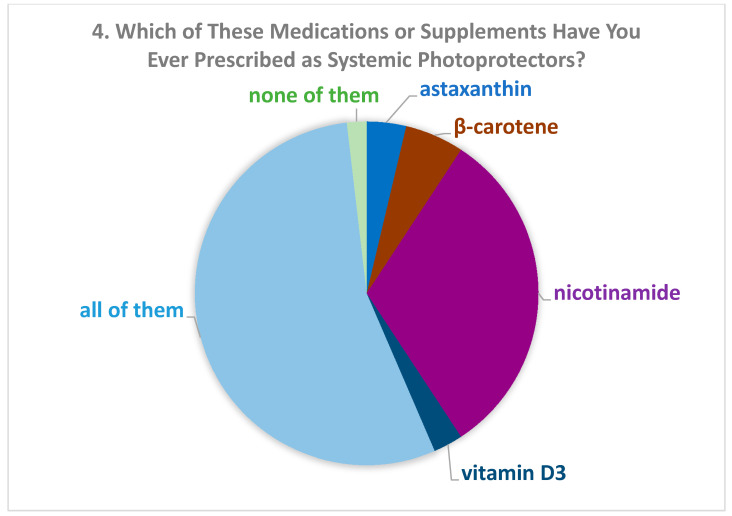
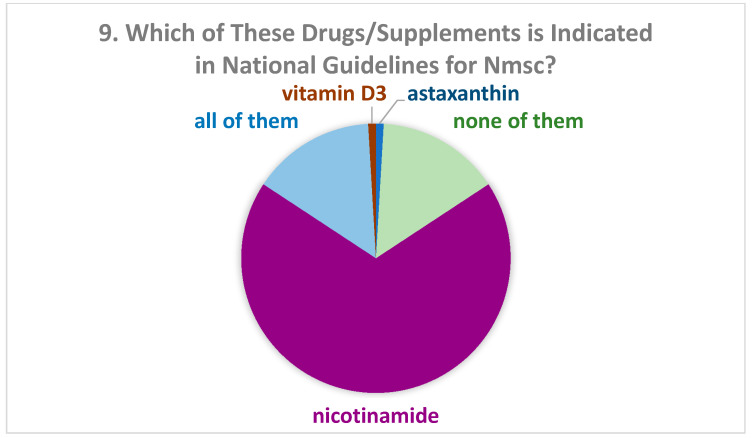
One hundred eight dermatologists answered the survey. There were 55 women (51.4%). The description of the study population is reported in Table 1. Concerning Question 1 (“Do you know any supplement or drug indicated in systemic photoprotection?”) only one participant gave a negative answer. Most of them (85.2%) believed that oral photoprotectors might have a role in the prevention of skin cancer (Question 2, Table 1), especially nicotinamide (34.3%) (Question 3, Table 1). Among participants who had finished training more recently, the percentage of those who believed in the role of oral photoprotectors in skin cancer prevention was significantly higher than in the other groups. More than half of dermatologists stated that they had prescribed all the drugs listed in the questionnaire (Question 4, Figure 1). Among those who had prescribed only one drug, the majority (31.5%) had prescribed nicotinamide. According to the responses to Question 5 (“How long do you usually prescribe photoprotectors?”), most of the dermatologists prescribed systemic photoprotectors for 2 to 3 months during summer (56.1%) or for 4 to 6 months (31.8%). Almost all of them (n = 106) associated such treatment with topical photoprotectors (Question 6: “Do you usually associate them with topical photoprotectors?”). More than 80% of dermatologists were aware of scientific studies which demonstrated an effect of systemic photoprotectors on NMSC (Question 7, Table 2). The proportions were very similar to those of Question 2 about the role of photoprotectors (Question 8, Table 2). Among all dermatologists, 68.5% thought that nicotinamide was indicated in national guidelines for NMSC (Figure 2, Question 9). Only 26.9% of participants reported being aware of scientific studies that had demonstrated the reduction of melanoma due to one of the products indicated in systemic photoprotection (Table 3, Question 10). Among them, 37.9% reported that the studies concerned vitamin D3, 17.2% β-carotene, 17.2% nicotinamide, 24.1% all listed drugs, and 3.4% none of them (Answer to Question 11: “If you answered “yes” to Question 10, which photoprotector was used?”). When asking which photoprotectors were indicated in guidelines for melanoma (Question 12), 44% answered vitamin D3, 17.2% nicotinamide, 3.4% β-carotene, 13.8% all drugs, and 20.7% none of them (results not shown).
Table 1.
Description of the characteristics of the dermatologists participating in the study and of their answers to the questions concerning their beliefs about oral photoprotectors.
| 2. Do You Believe That These Drugs or Supplements (Oral Photoprotectors) May Have a Role in the Prevention of Skin Cancers? | 3. If So, Which of These Drugs or Supplements Do You Believe May Play a Role in Systemic Photoprotection? Possible Answers: Astaxanthin, Β-Carotene, Nicotinamide, Vitamin D3, All of Them, None of Them. |
|||||||
|---|---|---|---|---|---|---|---|---|
| YES | β-Carotene | Nicotinamide | Vitamin D3 | All of Them | None of Them | |||
| N (column%) | N (row%) | N (row%) | N (row%) | N (row%) | N (row%) | N (row%) | ||
| Overall | 108 (100) | 92 (85.2) | 2 (1.9) | 37 (34.3) | 3 (2.8) | 63 (58.3) | 3 (2.8) | |
| Gender | Male | 52 (48.1) | 43 (82.7) | 0 | 19 (36.5) | 1 (1.9) | 29 (55.8) | 3 (5.8) |
| Female | 56 (51.9) | 49 (87.5) | 2 (3.6) | 18 (32.1) | 2 (3.6) | 34 (60.7) | 0 | |
| Years since end of training | <10 | 33 (30.6) | 32 (97.0) | 2 (6.1) | 10 (30.3) | 1 (3.0) | 20 (60.6) | 0 |
| 10–19 | 28 (25.9) | 26 (92.9) | 0 | 13 (46.4) | 2 (7.1) | 13 (46.4) | 0 | |
| ≥20 | 47 (43.5) | 34 (72.3) * | 0 | 14 (29.8) | 0 (0.0) | 30 (63.8) | 3 (6.4) | |
| Area | Northern | 24 (22.2) | 21 (87.5) | 1 (4.2) | 8 (33.3) | 3 (12.5) | 12 (50.0) | 0 |
| Central | 61 (56.5) | 55 (90.2) | 0 | 22 (36.1) | 0 (0.0) | 38 (62.3) | 1 (1.6) | |
| Southern | 23 (21.3) | 16 (69.6) ** | 1 (4.3) | 7 (30.4) | 0 (0.0) | 13 (56.5) | 2 (8.7) | |
| Workplace | Hospital | 17 (15.7) | 11 (64.7) *** | 0 | 5 (29.4) | 2 (11.8) | 10 (58.8) | 0 |
| University/research hospital | 47 (43.5) | 42 (89.4) | 0 | 19 (40.4) | 0 | 26 (55.3) | 2 (4.3) | |
| Local health department | 15 (13.9) | 14 (93.3) | 1 (6.7) | 6 (40.0) | 1 (6.7) | 7 (46.7) | 0 | |
| Private practice | 29 (26.9) | 25 (86.2) | 1 (3.4) | 7 (24.1) | 0 | 20 (69.0) | 1 (3.4) | |
* p < 0.001 from chi-square test compared with the categories “<10” and “10–19” grouped together. ** p < 0.05 from chi-square test compared with the categories “Northern” and “Central” grouped together. *** p < 0.01 from chi-square test compared with the other categories grouped together.
Figure 1.
Percentage of drugs or supplements prescribed by the dermatologists.
Table 2.
Description of the answers of dermatologists to the questions concerning their awareness of studies on the role of oral photoprotectors in nonmelanoma skin cancer (NMSC).
| 7. Are You Aware of Scientific Studies/Trials That Have Demonstrated the Reduction of NMSC Due to One of the Products Indicated in Systemic Photoprotection? | 8. If So, Which of These Drugs or Supplements? Possible Answers: Astaxanthin, β-Carotene, Nicotinamide, Vitamin D, All of Them, None of Them. |
||||||
|---|---|---|---|---|---|---|---|
| Yes | Nicotinamide | Astaxanthin | Vitamin D3 | All of Them | None of Them | ||
| N (row%) | N (row%) | N (row%) | N (row%) | N (row%) | N (row%) | ||
| Overall | 88 (81.5) | 74 (68.5) | 1 (0.9) | 1 (0.9) | 16 (14.8) | 16 (14.8) | |
| Gender | Male | 43 (82.7) | 33 (63.5) | 1 (1.9) | 0 | 9 (17.3) | 9 (17.3) |
| Female | 45 (80.4) | 38 (67.9) | 0 | 1 (1.8) | 9 (16.1) | 8 (14.3) | |
| Years since end of training | <10 | 30 (90.9) | 28 (84.8) | 0 | 0 | 3 (9.1) | 2 (6.1) |
| 10–19 | 26 (92.9) | 23 (82.1) | 1 (3.6) | 0 | 2 (7.1) | 2 (7.1) | |
| ≥20 | 32 (68.1) * | 20 (42.6) | 0 | 1 (2.1) | 13 (27.7) | 13 (27.7) | |
| Area | Northern | 19 (79.2) | 43 (70.5) | 0 | 0 | 4 (16.7) | 8 (13.1) |
| Central | 52 (85.2) | 16 (66.7) | 0 | 1 (1.6) | 9 (14.8) | 4 (16.7) | |
| Southern | 17 (73.9) | 12 (52.2) | 1 (4.3) | 0 | 5 (21.7) | 5 (21.7) | |
| Workplace | Hospital | 13 (76.5) | 11 (67.4) | 1 (5.9) | 0 | 2 (11.8) | 3 (17.6) |
| University/research hospital | 40 (85.1) | 34 (72.3) | 0 | 1 (1.2) | 6 (12.8) | 6 (12.8) | |
| Local health department | 11 (73.3) | 10 (66.7) | 0 | 0 | 2 (13.3) | 3 (20.0) | |
| Private practice | 24 (82.8) | 16 (55.2) | 0 | 0 | 8 (27.6) | 5 (17.2) | |
* p < 0.01 from chi-square test compared with the categories “<10” and “10–19” grouped together.
Figure 2.
Percentage of drugs or supplements which dermatologists believed to be indicated in national guidelines for NMSC.
Table 3.
Description of the answers of dermatologists to the question concerning their awareness of studies on the role of oral photoprotectors in melanoma.
| 10. Are You Aware of Scientific Studies/Trials That Have Demonstrated the Reduction of Melanoma Due to One of the Products Indicated in Systemic Photoprotection? | ||
|---|---|---|
| Yes (row%) | ||
| Overall | 29 (26.9) | |
| Gender | Male | 15 (28.8) |
| Female | 14 (25.0) | |
| Years since end of training | <10 | 12 (36.4) |
| 10–19 | 8 (28.6) | |
| ≥20 | 9 (19.1) | |
| Area | Northern | 10 (41.7) |
| Central | 12 (19.7) | |
| Southern | 7 (30.4) | |
| Workplace | Hospital | 7 (41.2) |
| University/research hospital | 12 (25.5) | |
| Local health department | 2 (13.3) | |
| Private practice | 8 (27.6) |
In the regression logistic model with Question 2 as the dependent variable, no significant association was found with any of the independent variables (results not shown). In Table 4 we report the results of the logistic regression model with Question 7 as the dependent variable. The association with the number of years since the end of dermatology training was significant. This means that dermatologists who had finished their training more recently (and in particular between 10 and 19 years) were more aware of studies on systemic photoprotectors for NMSC.
Table 4.
Results of the multivariable logistic regression model with Question 7 (“Are you aware of scientific studies/trials that have demonstrated the reduction of NMSC due to one of the products indicated in systemic photoprotection?”) as the dependent variable.
| Model | p Value | Exp (B) | 95.0% Confidence Interval for Exp (B) | ||||
|---|---|---|---|---|---|---|---|
| Levels | B | Standard Error | Lower Limit | Upper Limit | |||
| Gender | Female vs. male | −0.716 | 0.606 | 0.237 | 0.489 | 0.149 | 1.601 |
| Years since end of training | ≥30 (Ref) | -- | |||||
| <10 | 1.426 | 0.771 | 0.064 | 4.16 | 0.918 | 18.854 | |
| 10–19 | 2.101 | 0.899 | 0.019 | 8.177 | 1.405 | 47.58 | |
| 20–29 | −0.822 | 0.735 | 0.263 | 0.44 | 0.104 | 1.856 | |
| Geographical area | Southern (Ref) | -- | |||||
| Central | 0.784 | 0.756 | 0.299 | 2.191 | 0.498 | 9.632 | |
| Northern | 0.1 | 0.834 | 0.905 | 1.105 | 0.216 | 5.665 | |
| Workplace | Private practice (Ref) | -- | |||||
| Hospital | −0.002 | 0.888 | 0.998 | 0.998 | 0.175 | 5.685 | |
| University/research hospital | −0.084 | 0.709 | 0.905 | 0.919 | 0.229 | 3.687 | |
| Local health department | −1.084 | 0.863 | 0.209 | 0.338 | 0.062 | 1.835 | |
Ref = Reference category.
In Table 5 we report that only nicotinamide had an “A” grade of recommendation and a “1b” level of evidence considering clinical and preclinical studies from scientific literature in reducing the incidence of NMSC. Otherwise, β-carotene had a “D” grade of recommendation and a “1b” level of evidence. In contrast, vitamin D3 and astaxanthin presented a “D” grade of recommendation and a “5” level of evidence.
Table 5.
Clinical and preclinical studies, grade of recommendation, and level of evidence regarding different oral photoprotectors in the prevention of nonmelanoma skin cancer.
| Molecule | Studies Regarding Skin Cancer Prevention | Grade of Recommendation * | Level of Evidence * | |||||
|---|---|---|---|---|---|---|---|---|
| Preclinical Studies | Case Reports/Series | Observational Studies | Randomized Controlled Trials | Systematic Reviews/Meta-Analyses | Efficacy in Prevention of NMSC | |||
| Nicotinamide | + | − | − | + | − | + | A | 1b |
| β-Carotene | + | − | − | + | − | − | D | 1b |
| Vitamin D3 | + | − | + | − | + | − | D | 5 |
| Astaxanthin | + | − | − | − | − | − | D | 5 |
* From the Centre for Evidence-Based Medicine, https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed date: 30 December 2020). “+” means presence of studies while “−“ means no studies regarding that kind of research.
4. Discussion
In the present study, we observed that most of the included dermatologists considered that oral photoprotection, mainly nicotinamide, could have a role in the prevention of NMSC. Furthermore, more than half of them prescribed oral photoprotectors, again, mainly nicotinamide. Among participants, dermatologists who more recently finished training were more aware of the existence and the utilization of oral photoprotectors compared to older dermatologists. This likely depends on the fact that these treatments, and the relevant scientific studies, are quite recent. We also observed that dermatologists from Central and Northern Italy believed more in the role of oral photoprotection in the prevention of skin tumors than those from Southern Italy, while dermatologists working in hospitals believed less in photoprotection compared to dermatologists working in universities or in private practice. These data are probably also dependent on the age of participants. In fact, in the group from Southern Italy or working in hospitals we saw a higher percentage of dermatologists who had finished training in the past 20 years compared to the other areas and to the other workplaces. The multivariable model showed that dermatologists who had finished their training more recently were more aware of studies on the role of oral photoprotectors in NMSC than the older ones, taking into account gender, workplace, and geographical area. More than half of the dermatologists reported having prescribed all the drugs listed in the questionnaire, i.e., astaxanthin, β-carotene, vitamin D, and nicotinamide; however, among those who had prescribed only one drug, the majority had prescribed nicotinamide. Regarding the efficacy of systemic photoprotection in skin cancer prevention, many preclinical and clinical studies of different molecules have been reported, but only nicotinamide was demonstrated to play a role in reducing NMSC in humans [6,7,8]. Otherwise, the role in skin cancer prevention has been reported for the other molecules, mostly in preclinical studies, except β-carotene [9,11].
In our study, more than half of dermatologists prescribed systemic photoprotectors during summer, and almost one-third prescribed them for 4 to 6 months. A phase-three randomized controlled trial, carried out on 386 Australians, showed that oral nicotinamide treatment for 12 months (500 mg twice daily) was safe and effective in reducing the rates of new NMSC [7]. However, there is no evidence of benefit after nicotinamide is discontinued. It is thus very important to associate systemic photoprotection with topical photoprotectors, as was reported by almost all dermatologists in our study. Moreover, most dermatologists (81.5%) knew scientific studies which demonstrated an effect of systemic photoprotectors in NMSC prevention, mainly participants who had finished training more recently compared to the other groups. More than two-thirds of participants correctly answered that nicotinamide was the only product indicated in systemic photoprotection which had demonstrated a reduction of NMSC. Mainly dermatologists in the university and research hospitals and those who had finished training more recently answered correctly. It must be noted that considerable evidence indicates that oral nicotinamide is a photoprotective agent [13]. Nicotinamide prevents UV-induced ATP depletion, enhances UV-induced DNA repair, and mitigates the inflammation induced by environmental stressors in human keratinocytes [14]. Moreover, nicotinamide has photoprotective effects against carcinogenesis and immunosuppression in mice [15]. In human studies, a photoimmunoprotective role has been demonstrated for topical and oral nicotinamide administration. Indeed, it reduces the number of actinic keratoses [6] and protects against ultraviolet radiation-induced immunosuppression [16].
Furthermore, more than two-thirds of participants thought that nicotinamide was indicated in NMSC guidelines, confirming correct information. Specifically, nicotinamide is indicated as chemoprevention in the recent European guidelines on cutaneous squamous cell carcinoma [17,18]. On the other hand, it is not mentioned in the latest European guidelines on basal cell carcinoma, while it is mentioned in others [19,20,21]. Otherwise, about a quarter of participants confirmed they are aware of scientific studies reporting a reduction of melanoma due to systemic photoprotection, mainly vitamin D, and that this supplement was even indicated in guidelines for melanoma. A very recent study reported that nicotinamide shows a relevant antimelanoma activity in vitro and in vivo in mice, demonstrating that this molecule significantly delayed tumor growth in vivo and improved survival of melanoma-bearing mice [22]. However, no studies demonstrated that oral photoprotection has a role in melanoma prevention in humans, and no oral photoprotector is indicated in guidelines for melanoma.
The limitations of this study included the limited number of enrolled dermatologists, the necessary use of self-reported measures, and the setting circumscribed to the Italian population.
5. Conclusions
We found that most Italian dermatologists believed in the role of oral photoprotection in skin cancer prevention and more than half of them prescribed it. They were aware of the role of nicotinamide in the prevention of NMSCs, but there were still some doubts about the knowledge of oral photoprotection in melanoma prevention. Considering the importance of skin cancers, which present a progressive increase in incidence and an impressive cost of treatment, it is crucial to improve the knowledge of systemic photoprotection among dermatologists.
Acknowledgments
The authors thank the “Associazione Dermatologi Ospedalieri Italiani” (ADOI) for the collaboration in the survey.
Author Contributions
Conceptualization: L.F., F.S., and D.A.; writing—original draft preparation, L.F., F.S., D.A., M.H., and E.D.; writing—review and editing, L.F., F.S., F.R., D.A., M.H., E.D., A.P.(Andrea Paradisi), E.P., G.D.L., S.P., A.P.(Annarita Panebianco) and E.C.; funding acquisition, D.A., L.F., and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was mainly supported by the “Progetto Ricerca Corrente—RC4.3 2019” of the Italian Ministry of Health, Rome, Italy, and partially supported by AIRC grant (IF22206) to E.C., by “RF-2016-02362541” of the Italian Ministry of Health to E.D., and by Idi Farmaceutici s.r.l. “IDIFARM18-AB” to the Clinical Epidemiology Unit.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of IDI-IRCCS, Rome, Italy (Approval # 608-1).
Informed Consent Statement
Patient consent was “Not applicable”.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.González S., Gilaberte Y., Juarranz A. Oral and systemic photoprotection. In: Wang S.Q., Lim H.W., editors. Principles and Practice of Photoprotection. Springer International Publishing; Cham, Switzerland: 2016. pp. 387–403. [Google Scholar]
- 2.Lim H.W., Arellano-Mendoza M.I., Stengel F. Current challenges in photoprotection. J. Am. Acad. Dermatol. 2017;76:S91–S99. doi: 10.1016/j.jaad.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 3.Parrado C., Philips N., Gilaberte Y., Juarranz A., González S. Oral Photoprotection: Effective Agents and Potential Candidates. Front. Med. 2018;5:188. doi: 10.3389/fmed.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celleno L., Calzavara-Pinton P., Sala R., Arisi M.C., Bussoletti C. Photobiology, photodermatology and sunscreens: A comprehensive overview. Part 2: Topical and systemic photoprotection. G. Ital. Dermatol. Venereol. 2013;148:107–133. [PubMed] [Google Scholar]
- 5.Stahl W., Sies H. β-Carotene and Other Carotenoids in Protection from Sunlight. Am. J. Clin. Nutr. 2012;96:1179S–1184S. doi: 10.3945/ajcn.112.034819. [DOI] [PubMed] [Google Scholar]
- 6.Surjana D., Halliday G.M., Martin A.J., Moloney F.J., Damian D.L. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J. Investig. Dermatol. 2012;132:1497–1500. doi: 10.1038/jid.2011.459. [DOI] [PubMed] [Google Scholar]
- 7.Chen A.C., Martin A.J., Choy B., Fernández-Peñas P., Dalziell R.A., McKenzie C.A., Scolyer R.A., Dhillon H.M., Vardy J.L., Kricker A., et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015;373:1618–1626. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 8.Fania L., Mazzanti C., Campione E., Candi E., Abeni D., Dellambra E. Role of Nicotinamide in Genomic Stability and Skin Cancer Chemoprevention. Int. J. Mol. Sci. 2019;20:5946. doi: 10.3390/ijms20235946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green A.C., Williams G., Neale R., Hart V., Leslie D., Parsons P., Marks G.C., Gaffney P., Battistutta D., Frost C., et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: A randomized controlled trial. Lancet. 1999;354:723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- 10.Sies H., Stahl W. Carotenoids, and UV protection. Photochem. Photobiol. Sci. 2004;3:749–752. doi: 10.1039/b316082c. [DOI] [PubMed] [Google Scholar]
- 11.Dixon K.M., Norman A.W., Sequeira V.B., Mohan R., Rybchyn M.S., Reeve V.E., Halliday G.M., Mason R.S. 1α,25(OH)2 -vitamin D and a nongenomic vitamin D analogue inhibit ultraviolet radiation-induced skin carcinogenesis. Cancer Prev. Res. 2011;4:1485–1494. doi: 10.1158/1940-6207.CAPR-11-0165. [DOI] [PubMed] [Google Scholar]
- 12.Mahamat-Saleh Y., Aune D., Schlesinger S. 25-Hydroxyvitamin D status, vitamin D intake, and skin cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Sci. Rep. 2020;10:13151. doi: 10.1038/s41598-020-70078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen A.C., Damian D.L., Halliday G.M. Oral and systemic photoprotection. Photodermatol. Photoimmunol. Photomed. 2014;30:102–111. doi: 10.1111/phpp.12100. [DOI] [PubMed] [Google Scholar]
- 14.Surjana D., Halliday G.M., Damian D.L. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in human keratinocytes and ex vivo skin. Carcinogenesis. 2013;34:1144–1149. doi: 10.1093/carcin/bgt017. [DOI] [PubMed] [Google Scholar]
- 15.Surjana D., Halliday G.M., Damian D.L. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J. Nucleic Acids. 2010;2010:157591. doi: 10.4061/2010/157591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yiasemides E., Sivapirabu G., Halliday G.M., Park J., Damian D.L. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101–105. doi: 10.1093/carcin/bgn248. [DOI] [PubMed] [Google Scholar]
- 17.Stratigos A.J., Garbe C., Dessinioti C., Lebbe C., Bataille V., Bastholt L., Dreno B., Fargnoli M.C., Forsea A.M., Frenard C., et al. European Interdisciplinary Guideline on Invasive Squamous Cell Carcinoma of the Skin: Part 1. Epidemiology, Diagnostics and Prevention. Eur. J. Cancer. 2020;128:60–82. doi: 10.1016/j.ejca.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Stratigos A.J., Garbe C., Dessinioti C., Lebbe C., Bataille V., Bastholt L., Dreno B., Fargnoli M.C., Forsea A.M., Frenard C., et al. European Interdisciplinary Guideline on Invasive Squamous Cell Carcinoma of the Skin: Part 2 Epidemiology, Diagnostics and Prevention. Eur. J. Cancer. 2020;128:83–102. doi: 10.1016/j.ejca.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Peris K., Fargnoli M.C., Garbe C., Kaufmann R., Bastholt L., Seguin N.B., Bataille V., Marmol V.D., Dummer R., Harwood C.A., et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer. 2019;118:10–34. doi: 10.1016/j.ejca.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Lang B.M., Balermpas P., Bauer A., Blum A., Brölsch G.F., Dirschka T., Follmann M., Frank J., Frerich B., Fritz K., et al. S2k Guidelines for Cutaneous Basal Cell Carcinoma—Part 2: Treatment, Prevention and Follow-up. J. Dtsch. Dermatol. Ges. 2019;17:214–230. doi: 10.1111/ddg.13755. [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network . Guidelines Basal Cell Skin Cancer. National Comprehensive Cancer Network; Fort Washington, ML, USA: 2020. [(accessed on 6 May 2016)]. NCCN Clinical Practice Guidelines in Oncology. Version 1. Available online: https://jnccn.org/view/journals/jnccn/14/5/articlep574.xml. [Google Scholar]
- 22.Scatozza F., Moschella F., D’Arcangelo D., Rossi S., Tabolacci C., Giampietri C., Proietti E., Facchiano F., Facchiano A. Nicotinamide inhibits melanoma in vitro and in vivo. J. Exp. Clin. Cancer Res. 2020;39:211. doi: 10.1186/s13046-020-01719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.