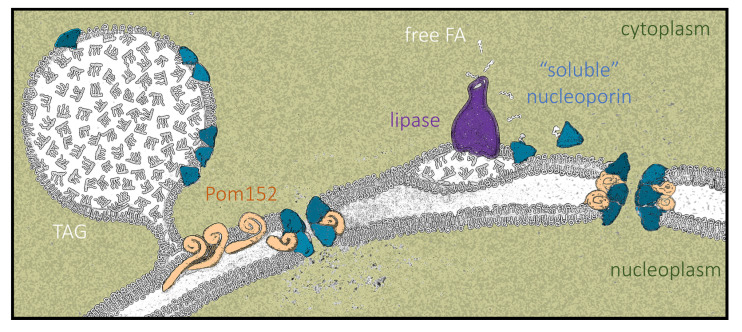
Figure 9.
Model of how LD status impacts Nup configurations at the nuclear pores. left: TriAcylGlycerols (TAG)-filled LD create a landing platform for nucleoporins, which can attach by their own features (i.e., amphipathic helices), by interaction with other Nups, or by congregation in the perimeter of LD, e.g., in the case of Pom152. This may titrate nucleoporins away from the nuclear pore, defining a particular NPC stoichiometry that matches a specific transport and chromatin landscape profile. right: In the event of LD consumption, lipase activity releases individual fatty acids. We propose that these FAs may bind core Nups of the inner pore channel, such as Nic96 and Nup157, promoting a conformational change that permits their dissociation from LD and migration to the pore. Simultaneously, the decreased surface of the LD upon shrinkage will naturally promote the eviction of other Nups, as ruled by protein crowding [53], as well as the diffusion back to the ER of transmembrane proteins congregating in the vicinity of the LD [66]. The availability of these components at the NPC will specify a different profile of transport and alternative chromatin features.