Abstract
Marine algae are a promising source of potent bioactive agents against oxidative stress, diabetes, and inflammation. However, the possible therapeutic effects of many algal metabolites have not been exploited yet. In this regard, we explored the therapeutic potential of Enteromorpha intestinalis extracts obtained from methanol, ethanol, and hexane, in contrasting oxidative stress. The total phenolic (TPC) and flavonoids (TFC) content were quantified in all extracts, with ethanol yielding the best values (about 60 and 625 mg of gallic acid and rutin equivalents per gram of extract, respectively). Their antioxidant potential was also assessed through DPPH•, hydroxyl radical, hydrogen peroxide, and superoxide anion scavenging assays, showing a concentration-dependent activity which was greater in the extracts from protic and more polar solvents. The α-amylase and α-glucosidase activities were estimated for checking the antidiabetic capacity, with IC50 values of about 3.8 µg/mL for the methanolic extract, almost as low as those obtained with acarbose (about 2.8 and 3.3 µg/mL, respectively). The same extract also showed remarkable anti-inflammatory effect, as determined by hemolysis, protein denaturation, proteinase and lipoxygenase activity assays, with respectable IC50 values (about 11, 4, 6, and 5 µg/mL, respectively), also in comparison to commercially used drugs, such as acetylsalicylic acid.
Keywords: oxidative stress, reactive oxygen species, antioxidant, polyphenols, diabetes, anti-inflammatory, marine algae, seaweed
1. Introduction
Secondary metabolites, such as polyphenols, carotenoids, terpenes, and alkaloids are gaining an ever-increasing attention because of their antioxidant capacity and the consequent beneficial effect on the human body [1,2]. These bioactive compounds have been widely investigated in plants and fruits, but they still represent a largely unexplored source of phenolic and flavonoid compounds in marine algae. Their extraction is usually accomplished in organic solvents, such as petroleum ether, hexane, chloroform, ethyl acetate, acetone, and methanol. Nevertheless, cheap and non-toxic solvents are preferred from an industrial point-of-view. In addition, alcoholic extracts generally allow a better extraction of polyphenols [3]. Because of their well-known beneficial health effects, as well as their positive influence on several pathologies, such as cancer, diabetes, cardiovascular, neurodegenerative, and inflammatory diseases, and in order to improve their pharmacokinetics, secondary metabolites are also being exploited in more technological formulations [4,5].
Marine algae have been extensively used as food for humans and animals. Macroalgae are rich in minerals, vitamins, polysaccharides, and other bioactive substances, such as proteins, lipids, carotenoid, and polyphenols. These latter compounds are produced by marine algae and sea grasses primarily for protective, structural, and ecological objectives; nevertheless, they also confer interesting properties to their raw material, such as antioxidant, antidiabetic, anti-inflammatory, antiviral, antibacterial, and other disease-preventive agents [6]. The beneficial health effects of marine macroalgae are largely related to their radical-scavenging ability, which can reduce oxidative stress. The commercialization of seaweed is based on their use as food supplements and nutraceuticals, ultimately developing non-toxic functional products with antioxidant properties.
Green seaweeds have been shown to possess strong antioxidant properties due, among others, to the contained flavonoids, bromophenols, and chlorophylls [7,8]. Among them, the Enteromorpha genus belongs to the class Chlorophyceae and it represents a rich source of secondary metabolites. For example, alcoholic extracts from E. compressa were recently shown to possess strong antioxidant capacity, also because of the high content of phenols and flavonoids (about 98 and 562 mg of gallic acid and rutin equivalents per gram of dry weight of extract, respectively) [9]. Interestingly, Wekre and colleagues studied the polyphenolic content in E. intestinalis by different techniques, e.g., NMR and RP-HPLC, also determining the major constituents by mass analysis [10]. Nevertheless, this group of algae also possess a variety of additional health-beneficial effects, e.g., anti-tumoral, antibacterial, and antidiabetic, that deserve further investigation [11,12,13,14,15].
Diabetes mellitus is a chronic metabolic disorder characterized by high blood glucose levels and it can lead to renal dysfunction, cardiovascular diseases, and retinal damage [16]. Commercially available antidiabetic drugs exert several adverse side effects, pushing the research for novel natural drugs. Marine algal bioactive compounds were able to inhibit α-amylase, α-glucosidase, dipeptidyl peptidase-4, aldose reductase, and protein tyrosine phosphatase 1B (PTP 1B) enzymes, which play a key role in the digestion of carbohydrate, thus leading to delayed glucose absorption in blood and plasma [17]. Marine algal compounds were also able to modulate GLUT-4 and AMPK signaling pathways and trigger glucose tolerance. The α-amylase inhibition delays the assimilation process by hindering the breakdown of starch and can be used as a target for treatment against hyperglycemic disorder [17].
Inflammation is usually referred to as an intricate series of biological reactions of the vascular tissues subjected to deleterious stimuli. In addition, inflammation is also associated with pain that leads to protein denaturation, increase of membrane modifications, and vascular permeability [18]. Non-steroidal anti-inflammatory drugs (NSAIDs) have been extensively used as anti-inflammatory agents although they present several adverse effects, such as gastric irritation, which can lead to gastric ulcer [19]. The marine environment is rich of both micro- and macro-algal biodiversity, which could contain novel therapeutic compounds [20]. Hence, the identification and screening of natural bioactive compounds from marine algal source with anti-inflammatory activity is promising.
Although some studies already described the antioxidant capacity of marine algae, the ability of bioactive compounds in Enteromorpha intestinalis in fighting free radicals and related diseases, e.g., diabetes and inflammatory diseases, are yet to be investigated.
The aim of this study was to investigate the ability of methanol, ethanol, and hexane extracts of Enteromorpha intestinalis in scavenging free radicals through several in vitro assays, such as DPPH•, hydroxyl radical, H2O2 radical, and superoxide anion radical scavenging assay. In addition, a preliminary investigation of their anti-diabetic activity was performed via α-amylase and α-glucosidase inhibition assays. On the other hand, inhibition of albumin denaturation, antiproteinase activity, membrane stabilization, and anti-lipoxygenase activity were used to evaluate the potential anti-inflammatory capacity of the extracts as compared to commercial acetylsalicylic acid.
2. Results
2.1. Solvent Extracts of E. intestinalis Are Promising Source of Antioxidative Phytochemicals
Three different solvents were tested for the extraction of the secondary metabolites from E. intestinalis, namely methanol, ethanol, and hexane, yielding the corresponding MEEI, EEEI, and HEEI extracts. A qualitative phytochemical analysis revealed the presence of glycosides, alkaloids, coumarin, terpenoids, proteins, phenols, and tannins (Table 1).
Table 1.
Presence (+) or absence (−) of several phytochemical classes in Enteromorpha intestinalis extracts using different solvents.
| Bioactive Compounds | MEEI | EEEI | HEEI |
|---|---|---|---|
| Alkaloids | + | + | + |
| Glycosides | + | + | + |
| Reducing sugars | − | − | − |
| Proteins | + | + | + |
| Terpenoids | + | + | + |
| Phenols and Tannins | + | + | + |
| Steroids | − | − | − |
| Saponins | + | − | − |
| Anthocyanins | − | − | − |
| Coumarin | + | + | + |
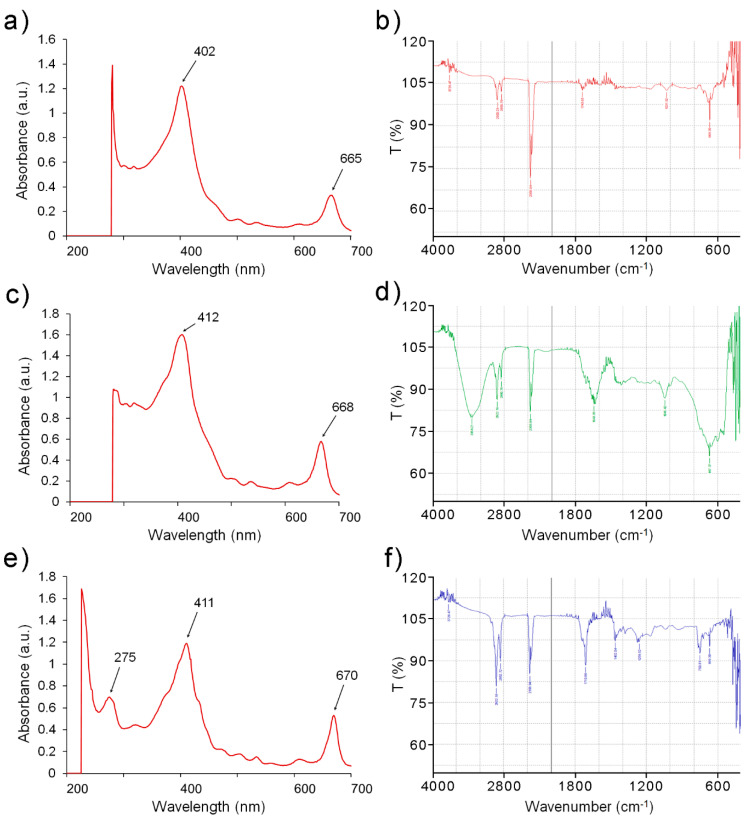
No steroids, anthocyanins, and reducing sugars were recovered by any solvent, while saponins were detected only in the methanolic extract. The qualitative test was followed by a UV-visible spectrophotometric analysis that evidenced absorption peaks between 260–290, 380–460, and 650–680 nm (Figure 1a,c,e).
Figure 1.
UV-Vis (a,c,e) and FT-IR (b,d,f) spectral analyses of the extracts of Enteromorpha intestinalis obtained by methanol (a,b), ethanol (c,d), and hexane (e,f). The analyses evidenced minor differences among the spectra corresponding to the different concentration and composition of the bioactive functional groups extracted.
The presence of functional groups typical from flavonoids and other phenolic compounds was also established by FT-IR analysis (Figure 1b,d,f), and the major peaks observed in each extract are summarized in Table 2.
Table 2.
Functional groups and bonding patterns in the methanolic, ethanolic, and hexane extracts of Enteromorpha intestinalis as determined by FT-IR analysis.
| Extract | Peak Value (cm−1) | Functional Group | Bonding Pattern |
|---|---|---|---|
| MEEI | 3726.47 | Alcohol (free) | O-H stretching |
| 2920.23 | Alkane | C-H stretching | |
| 2850.79 | Alkane | C-H stretching | |
| 2358.94 | Carbon dioxide | O=C=O | |
| 1743.65 | Esters (6-membered lactone) | C=O stretching | |
| 1031.92 | Sulfoxide | S=O stretching | |
| 669.30 | Halo compound | C-Br | |
| EEEI | 3354.21 | Alcohol (intermolecular bond) | O-H stretching |
| 2922.16 | Alkane | C-H stretching | |
| 2850.79 | Alkane | C-H stretching | |
| 2358.94 | Carbon dioxide | O=C=O | |
| 1643.35 | Alkene (cis-disubstituted) | C=C stretching | |
| 1045.42 | Sulfoxide | S=O stretching | |
| 667.37 | Halo compound | C-Br | |
| HEEI | 3726.47 | Alcohol (free) | O-H stretching |
| 2922.16 | Alkane | C-H stretching | |
| 2852.72 | Alkane | C-H stretching | |
| 2358.94 | Carbon dioxide | O=C=O | |
| 1710.86 | Conjugated acid | C=O stretching | |
| 1462.04 | Sulfate | S=O stretching | |
| 1259.52 | Alkyl aryl ether | C-O stretching | |
| 750.31 | Monosubstituted | C-H bending | |
| 669.30 | Halo compound | C-Br |
To obtain a quantitative indication of the presence of these secondary metabolites, the TPC and TFC contents in MEEI, EEEI, and HEEI were determined. The TPC was found to be higher in ethanolic extract (about 60 mg of GAE/g) while the lowest values were obtained from the hexane extract. Similarly, the TFC ranged from about 625 mg of RUE/g in EEEI to about 152 mg of RUE/g in the hexane extract. Much lower amounts of ascorbic acid were detected in all extracts, although with a similar trend. The total antioxidant capacity (TAC) exerted by the ethanolic extracts was quantified to be about 177 mg of AAE/g, as determined by the phosphomolybdenum assay. Again, the lowest TAC was observed in the hexane extract (about 45 mg of AAE/g) (Table 3).
Table 3.
Total phenolic, flavonoid, and ascorbic acid content, and total antioxidant capacity in Enteromorpha intestinalis extracts.
| Assay | MEEI | EEEI | HEEI |
|---|---|---|---|
| Total phenolic content (mg of GAE 1/g) |
23.00 ± 0.05 * | 59.67 ± 0.01 **++ | 11.91 ± 0.06 *+ |
| Total flavonoid content (mg of RUE 2/g) |
416.00 ± 0.03 ## | 624.67 ± 0.05 ##++ | 152.00 ± 0.14 #+ |
| Ascorbic acid content (µg of AA/g) |
336.00 ± 0.36 $ | 446.00 ± 0.43 $$++ | 195.00 ± 0.33 $+ |
| Total antioxidant activity (mg of AAE 3/g) |
88.00 ± 0.02 & | 177.33 ± 0.02 &&++ | 45.00 ± 0.04 &+ |
1 GAE: gallic acid equivalents; 2 RUE: rutin equivalents; 3 ascorbic acid equivalents. Relative levels of significance were determined by comparing the extracts to respective standards in the assays; * represents the significance of TPC with gallic acid, # represents the significance of TFC with rutin, $ represents the significance of AA content with ascorbic acid, & represents the significance of TAA with ascorbic acid equivalent. The + represents level of significance between either EEEI or HEEI with respect to MEEI.
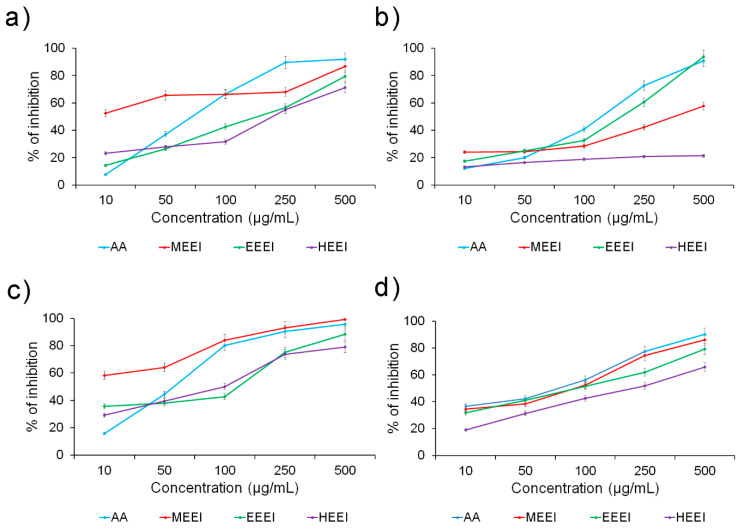
Phenols and flavonoids exhibit potent antioxidant and free radical scavenging activity. The ROS-associated free radical scavenging properties of MEEI, EEEI, and HEEI were measured by DPPH•, hydroxyl radicals, hydrogen peroxide, and superoxide radicals scavenging assay in the range 10–500 µg/mL and compared to ascorbic acid (AA), used as reference (Figure 2).
Figure 2.
Antioxidant scavenging capacity of the different solvent extracts from Enteromorpha intestinalis as determined by (a) DPPH• radical scavenging activity, (b) hydrogen peroxide (H2O2) scavenging activity, (c) hydroxyl (•OH) ion radical scavenging activity, and (d) superoxide (•O2) scavenging activity assays.
In general, an increasing antioxidant effect was noted by increasing the extracts’ concentration in all assays. In the DPPH• radical scavenging assay, the MEEI extract exhibited the highest scavenging activity at lower concentrations (up to 50 µg/mL) in comparison to the other extracts and ascorbic acid, although this difference diminished at higher concentrations (Figure 2a). MEEI and even more EEEI also had better ability to scavenge H2O2 radicals compared to HEEI, especially at concentrations above 100 µg/mL (Figure 2b). The MEEI extract presented the best inhibition profile in the hydroxyl ion radical assay, exhibiting even better values than ascorbic acid, especially at concentrations below 100 µg/mL (Figure 2c). On the other hand, much closer data were obtained when evaluating the superoxide radical scavenging potency, although the HEEI extract showed again the worst inhibition values (Figure 2d).
Similar results were obtained by comparing the IC50 values, where the MEEI extract showed very low inhibitory concentrations in the DPPH• and hydroxyl radical scavenging assays (about 0.5 and 0.3 µg/mL, respectively), also in comparison to ascorbic acid (about 2.6 and 2.3 µg/mL, respectively) (Table 4). Satisfactory values were also obtained by the EEEI extract, with concentrations comparable to that of ascorbic acid in the H2O2 scavenging assay (IC50 values of about 3.2 and 3.1 µg/mL, respectively). On the other hand, the extract from the apolar hexane yielded the worst IC50 values in all assays.
Table 4.
IC50 values (in µg/mL) obtained from the free radical scavenging assays with ascorbic acid and the Enteromorpha intestinalis extracts.
| Assay | Ascorbic Acid | MEEI | EEEI | HEEI |
|---|---|---|---|---|
| DPPH• scavenging activity | 2.62 ± 0.23 | 0.50 ± 0.01 ** | 3.38 ± 0.57 * | 3.68 ± 0.98 * |
| H2O2 scavenging activity | 3.13 ± 0.87 | 4.72 ± 0.67 * | 3.22 ± 0.68 * | 18.41 ± 1.78 ** |
| Hydroxyl radical scavenging activity | 2.26 ± 0.22 | 0.32 ± 0.01 ** | 2.58 ± 0.11 * | 2.68 ± 0.87 * |
| Superoxide scavenging activity | 2.26 ± 0.67 | 2.49 ± 0.32 * | 2.73 ± 0.16 * | 3.69 ± 0.73 * |
Relative levels of significance were determined by comparing the extracts to ascorbic acid.
The correlation between TPC and antioxidant activity was calculated through linear regression for all the extracts. As expected, high correlation coefficients were obtained in all cases (R2(DPPH•) = 0.93 and R2(H2O2) = 0.99 for the ethanolic extract; R2(DPPH•) = 0.87 and R2(H2O2) = 0.99 for the methanolic extract; R2(DPPH•) = 0.97 and R2(H2O2) = 0.68 for the hexane extract) confirming the important role of polyphenols in contrasting the oxidative stress.
2.2. Solvent Extracts of E. intestinalis Exhibit Potential Antidiabetic Activity
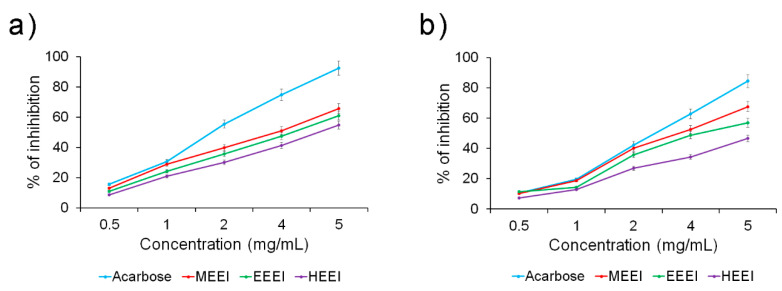
To determine the antidiabetic property of the solvent extracts of E. intestinalis, their ability to inhibit α-amylase and α-glucosidase activities were investigated. MEEI, EEEI, and HEEI were shown to have a concentration-dependent inhibitory effect on the α-amylase activity, with the MEEI extract displaying the best inhibitory effect (Figure 3a).
Figure 3.
Antidiabetic capacity of different solvent extracts from Enteromorpha intestinalis as determined by (a) α-amylase, and (b) α-glucosidase inhibitory activity assays.
The α-glucosidase activity also followed a similar pattern, although the difference between the MEEI extract and the other ones was even more pronounced (Figure 3b). The potent α-amylase and α-glucosidase activity of the MEEI extract was confirmed by very good IC50 values (about 3.8 µg/mL in both assays), quite close to those obtained with acarbose, used as reference (IC50 of about 2.8 and 3.3 µg/mL, respectively) (Table 5). Slightly higher values were obtained by EEEI and, especially, HEEI extracts.
Table 5.
IC50 values (in µg/mL) of the antidiabetic activity of the extracts of Enteromorpha intestinalis.
| Assay | Acarbose | MEEI | EEEI | HEEI |
|---|---|---|---|---|
| α-Amylase activity | 2.81 ± 0.78 | 3.81 ± 0.45 * | 4.15 ± 0.56 * | 4.68 ± 0.33 * |
| α-Glucosidase activity | 3.32 ± 0.96 | 3.82 ± 0.56 * | 4.32 ± 0.78 * | 5.44 ± 0.51 * |
Relative levels of significance were determined by comparing the extracts to acarbose.
2.3. Solvent Extracts of E. intestinalis Are Promising Sources of Anti-Inflammatory Compounds
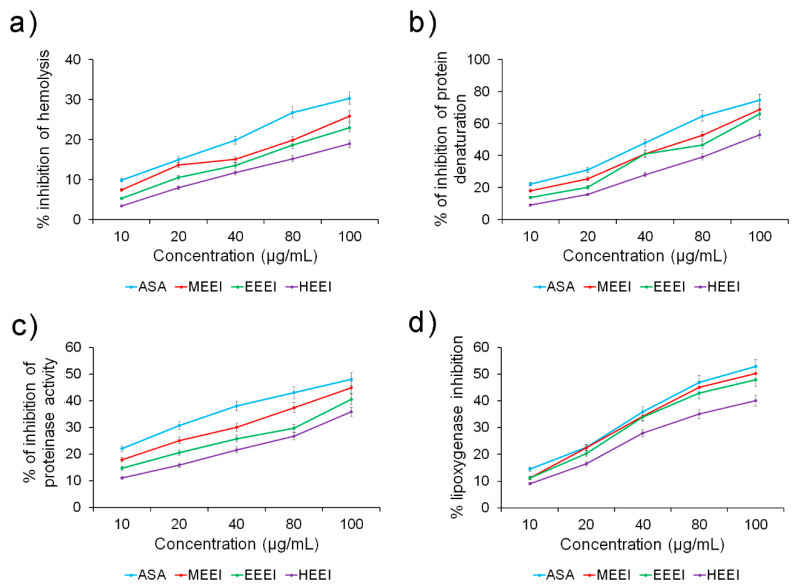
Four assays were performed to determine the anti-inflammatory capacity of the E. intestinalis extracts, namely hemolysis, protein denaturation, proteinase activity, and lipoxygenase activity. All extracts were able to inhibit all the activities in a concentration-dependent manner (Figure 4). Again, the MEEI extract was the best inhibition, followed by EEEI. The difference between the two polar extracts was almost non-existent in inhibiting lipoxygenase, where they also displayed an activity comparable to that of acetylsalicylic acid (ASA), used as reference (Figure 4d). On the other hand, the HEEI extract resulted to be the least powerful inhibitor at all the tested concentrations.
Figure 4.
Anti-inflammatory capacity of different solvent extracts from E. intestinalis as determined by (a) hemolysis assay, (b) protein denaturation, (c) proteinase inhibitory activity, and (d) lipoxygenase inhibitory activity.
The corresponding IC50 values were estimated by linear regression in all assays, confirming the very good performance of the MEEI extract, followed closely by EEEI and, to a greater extent, by HEEI (Table 6). The IC50 values of the alcoholic extracts were almost comparable to those obtained by ASA, especially in inhibiting protein denaturation, proteinase activity, and lipoxygenase activity (IC50 values of about 3.7, 5.8, and 4.7 µg/mL for MEEI compared to 3.1, 5.1, and 4.5 for ASA, respectively). On the other hand, greater differences were noted in inhibiting hemolysis compared to ASA (IC50 value of about 8.6 µg/mL), although the extracts still exhibited satisfactory values, especially MEEI (IC50 value of about 10.8 µg/mL).
Table 6.
IC50 values (in µg/mL) of the anti-inflammatory activity of the extracts of Enteromorpha intestinalis.
| Assay | Acetylsalicylic Acid | MEEI | EEEI | HEEI |
|---|---|---|---|---|
| Hemolysis inhibition activity | 8.62 ± 0.99 | 10.81 ± 1.12 * | 11.21 ± 1.76 * | 13.09 ±1.98 * |
| Protein denaturation activity | 3.14 ± 0.88 | 3.68 ± 0.81 * | 3.95 ± 0.19 * | 4.88 ± 0.49 * |
| Proteinase activity | 5.11 ± 0.97 | 5.84 ± 0.91 * | 6.89 ± 0.37 * | 7.60 ± 0.12 * |
| Lipoxygenase activity | 4.52 ± 0.58 | 4.72 ± 0.76 * | 4.95 ± 0.86 * | 6.01 ± 0.27 * |
Relative levels of significance were determined by comparing the extracts to ASA.
3. Discussion
Recently, phytochemicals have attained great attention for the screening and development of novel therapeutic pharmacophores [21]. Algae-based bioactive compounds, such as alkaloids, carbohydrates, proteins, glycosides, flavonoids, phenols, saponins, tannins, coumarins, and terpenoids, have shown promising in vitro results and could be extensively used as therapeutic agents against several diseases [17]. The present investigation was designed to understand the in vitro antioxidant potency of different solvent extracts of Enteromorpha intestinalis, a marine alga typical from Chilika lagoon, in India, with particular attention to their antidiabetic and anti-inflammatory activity. Three solvents with different polarity and physico-chemical characteristics, namely methanol, ethanol, and hexane, were used to extract many secondary metabolites from the plant. More in detail, alkaloids, glycosides, steroids, coumarins, terpenoids, proteins, coumarins, phenols, and tannins were detected in all extracts, while saponins were present only in the methanolic one, thus contributing to their antioxidant and disease-preventing potency [9].
Flavonoids, and polyphenols in general, are abundantly present in many plants, fruits, and derived products, and they exert an antioxidant effect by neutralizing free radicals [22,23,24,25]. The UV-visible and the FT-IR spectrophotometric analyses also yielded the typical signals of phenolic compounds, thus confirming their presence also in the E. intestinalis extracts [26]. The results of our study are in the line with the findings from a previous study in Padina pavonica [27] and Sargassum wightii seaweed extracts [28]. A quantitative analysis revealed that the highest content of phenols and flavonoids was present in the EEEI (about 60 and 625 mg of GAE and RUE per gram of extract for TPC and TFC, respectively), closely followed by the MEEI (about 23 and 416 mg of GAE and RUE per gram of extract for TPC and TFC, respectively). On the other hand, the hexane extract contained smaller amounts (about 12 and 152 mg of GAE and RUE per gram of extract for TPC and TFC, respectively), probably because the apolar solvent was not as efficient as the protic solvents in extracting these molecules. These data are in line with previous findings on the E. compressa, were the alcoholic solvents also extracted slightly higher but still comparable amounts of TPC and TFC (about 98 and 562 mg of GAE and RUE per gram of extract, respectively) [9]. The higher content of polyphenols in the ethanolic extract also resulted in the best TAC (about 177 mg of AAE per gram of extract), somewhat lower compared to the corresponding methanolic extract from E. compressa (about 339 mg of AAE per gram of extract) [9].
The aberrant accumulation of H2O2 is liable for oxidative stress [29,30] and inflammatory reactions, which can contribute to the development of pathological conditions, such as cancer, diabetes, and cardiovascular diseases [31]. The rapid decomposition of H2O2 subsequently generate hydroxyl radicals (•OH) that initiate lipid peroxidation and cellular damage [32]. The present study revealed potent inhibition of H2O2 radicals by the extracts in a concentration-dependent manner, with lower IC50 values obtained from the alcoholic extracts (about 4.7 and 3.2 µg/mL for the MEEI and EEEI extracts, respectively) compared to that from the apolar one (about 18.4 µg/mL). This data is in agreement with that achieved from the methanolic extract from E. compressa (about 2.8 µg/mL) [9] but considerably lower than that from the root of Asparagus racemosus Linn (about 27.6 µg/mL) [33]. Superoxide anion radicals are well known initiators of hydrogen peroxide or singlet oxygen in living systems [34]. Again, all the extracts from E. intestinalis displayed good superoxide radical scavenging activity in a concentration-dependent manner but with lower IC50 values for MEEI and EEEI (about 2.5 and 2.7 µg/mL, respectively) compared to HEEI (3.69 µg/mL) and confirming our previous findings [9,35]. A similar trend was also obtained by the DPPH• and in the hydroxyl radical scavenging assays. DPPH• is a stable organic free radical, which loses its absorption by accepting an electron or a free radical species. By getting an electron from an antioxidant molecule, DPPH• becomes diamagnetic by reduction into hydrazine derivative [36]. The MEEI extract presented very low IC50 values (about 0.5 and 0.3 µg/mL for the DPPH• and the •OH scavenging assays, respectively) performing significantly better than ascorbic acid (about 2.6 and 2.3 µg/mL for the DPPH• and the •OH scavenging assays, respectively) and compared to the extracts from E. compressa (about 3.2 and 2.9 µg/mL, respectively) and Asparagus racemosus Linn (about 9.9 and 169.3 µg/mL, respectively) [33].
Diabetes mellitus (DM) is a metabolic disorder that affects the global population in the modern era [37,38]. The acute phase of diabetes is correlated with oxidative stress, which triggers nephropathy, retinopathy, and neuropathy [16]. However, hyperglycemia can be suppressed via α-amylase inhibition, which allows D-glucose absorption into the bloodstream [39]. Acarbose has been found to be an efficient drug against type 2 diabetes by suppressing the hydrolysis of carbohydrates [40]. MEEI, EEEI, and HEEI exhibited potential anti-diabetic effects, as suggested by their ability to inhibit α-amylase activity with low IC50 values (about 3.8, 4.2, and 4.7 µg/mL, respectively), although slightly higher than that from acarbose at the same concentration (about 2.8 µg/mL) [41]. Nevertheless, this difference was less marked when testing the α-glucosidase inhibitory activity, especially for the MEEI extract (IC50 of about 3.8 and 3.3 µg/mL for MEEI and acarbose, respectively).
Leukocytes play a pivotal role in cellular infiltration during inflammation process by releasing lysosomal enzymes, such as proteases, which cause tissue damage [42]. Furthermore, inflammation triggers secondary damages via free radical-induced lipid peroxidation. As the lysosomal membrane is similar to red blood cell membrane, hemolysis inhibition provides a good insight into the inflammation process. In fact, the stabilization of the cell membrane inhibits the lysis and release of cytoplasmic material, thus stopping the inflammation [43]. The MEEI was the most efficient in protecting against heat-induced hemolysis, although less successfully than ASA (IC50 of about 10.8 and 8.6 µg/mL, respectively) but better than an aqueous extract from Albuca setosa (14% inhibition at 125 µg/mL) [42]. All E. intestinalis extracts, especially the MEEI, also inhibited protein denaturation, a typical process characterizing inflammation, with low IC50 values (about 3.7 and 3.1 µg/mL for MEEI and ASA, respectively), much more efficiently than a water extract from the stem of Wedelia trilobataon (about 51.1 µg/mL) [44]. Similar results were obtained in inhibiting proteinase and lipoxygenase activity. Lipoxygenases are key enzymes which catalyze deoxygenation of polyunsaturated fatty acids to produce essential mediators of cis- and trans-conjugated diene hydroperoxides, such as the leukotrienes. The EEEI and especially the MEEI extracts possessed high concentration-dependent proteinase and lipoxygenase inhibitory activities, as already observed in previous studies with Leptadenia pyrotechnica and Mahonia aquifolium extracts [45,46], with IC50 values (about 4.7, 5.0, and 6.0 µg/mL for MEEI, EEEI, and HEEI, respectively) almost comparable to that of acetylsalicylic acid (about 4.5 µg/mL), thus confirming their potential anti-inflammatory effect.
4. Materials and Methods
4.1. Sampling
A sample of the marine macroalgae Enteromorpha intestinalis was collected in June 2019 by hand picking from the Chilika lagoon (Kalijai), in India (longitude 19°39.934′ N, latitude 85°13.033′ E). After morphological characterization, the characteristics of the sample were compared with those in the monograph of marine algae and the alga identified as Enteromorpha intestinalis [47]. The sample was 25 cm in height and its cells were 10–14 µm long and 6–10 µm wide. The specimen was deposited in the Herbarium of the Department of Botany, Berhampur University, Odisha (India), and assigned the herbarium number BU1919.
4.2. Extract Preparation
The sample was dried at 80 °C in an oven, crushed to powder with a mechanical grinder, and extracted through a Soxhlet apparatus. After the extraction in different solvents, the filtrate sample was subjected to evaporation in a rotary evaporator and the % of yield of E. intestinalis calculated. The three solvent extracts, i.e., the methanolic extract of E. intestinalis (MEEI), the ethanolic extract of E. intestinalis (EEEI), and the hexane extract of E. intestinalis (HEEI), were prepared as already described [35]. The dried extracts were kept at 4 °C before use.
4.3. UV Visible and FT-IR Spectroscopy
The UV-visible spectra of the different solvent extracts from E. intestinalis were recorded from 200 to 700 nm on an Eppendorf BioSpectrometer 6136 spectrophotometer. FT-IR analyses were recorded using a Nicolet FT-IR spectrophotometer in transmittance mode from 400 to 4000 cm−1.
4.4. Qualitative Phytochemical Screening
Qualitative analysis of MEEI, EEEI, and HEEI extracts was performed to conclude the presence of various bioactive metabolites using the standard qualitative procedures as already reported elsewhere [35].
4.5. Estimation of Total Phenol Content and Total Flavonoids
The total phenolic content of the MEEI, EEEI, and HEEI was estimated by the Folin–Ciocalteu method by measuring the optical density (OD) at 765 nm, as already reported [48]. Gallic acid was used as internal standard. The results were expressed as milligram of gallic acid equivalent dry weight of the sample per 1 g of extract (mg of GAE/g).
4.6. Estimation of Total Flavonoid Content
The spectrophotometric determination of total flavonoid content was estimated by measuring the OD at 415 nm, as already reported [49]. Rutin was used as internal standard. The TFC of the extract was expressed in terms of rutin equivalent dry weight of the algal sample per 1 g of extract (mg of RUE/g).
4.7. Estimation of Ascorbic Acid Content
The ascorbic acid content was estimated following a procedure by Al-Ani et al. [50]. Briefly, the crude extract (1 mL) was mixed with 2,4-nitro-phenylhydrazine reagent (1 mL) and incubated at 95 °C for 15 min. Then, H2SO4 (85%, 5 mL) was added dropwise to the reaction mixture in ice cold condition. After 30 min of incubation, the absorbance was measured at 520 nm with an Eppendorf BioSpectrometer 6136 (Eppendorf AG, Hamburg, Germany). Ascorbic acid was used as standard and its content in the extracts was expressed in terms of µg of AA per 1 g of extract (µg of AA/g).
4.8. Determination of Free Radical Scavenging Activity
4.8.1. 1,1-Diphenyl-2-picrylhydrazyl Radical (DPPH•) Scavenging Activity
The DPPH• free radical scavenging activity of the MEEI, EEEI, and HEEI extracts was determined as already described (OD at 517 nm) by using ascorbic acid as standard [35]. The DPPH• decolorization was measured and expressed as % of inhibition.
4.8.2. Hydroxyl Radical Scavenging Activity
The hydroxyl radical scavenging efficacies of different solvent extracts were determined as already described (OD at 562 nm) by using ascorbic acid as standard [35]. The scavenging potency was calculated and represented as % of inhibition.
4.8.3. Superoxide Anion Radical Scavenging Activity
The superoxide anion radical scavenging efficacy of the different solvent extracts was determined as already described (OD at 560 nm) by using ascorbic acid as standard [9]. The scavenging potency was measured and represented as % of inhibition.
4.8.4. Hydrogen Peroxide Radical Scavenging Activity
The hydrogen peroxide radical scavenging efficacy of the different solvent extracts was determined as already reported (OD at 230 nm) by using ascorbic acid as the standard [51]. The scavenging potency was measured and represented as % of inhibition.
4.8.5. Total Antioxidant Activity by Phosphomolybdenum Assay
The total antioxidant activity of different solvent extracts was determined by measuring the OD at 695 nm, as already reported [22]. Ascorbic acid was used for preparing the calibration curve. The total antioxidant activity of the extracts was expressed as the number of gram equivalents of ascorbic acid.
4.9. Antidiabetic Activity
4.9.1. Determination of α-Amylase Inhibition
The α-amylase inhibitory activity was measured according to previously published procedures [39,52]. Briefly, stock sample solutions were prepared (10 mg/mL) and further diluted with 20 mM phosphate buffer saline (PBS) at pH 6.9 to a final range of 0.5–5.0 mg/mL. The sample (20 μL) was mixed with 20 mM PBS (20 μL, pH 6.9) and 1% starch solution (20 μL). The reaction mixture was incubated in an orbital shaker (37 °C, 3 min) followed by the addition of porcine pancreatic α-amylase solution (20 μL). The reaction was incubated again (37 °C, 15 min). The reaction was then stopped by adding 1 M HCl (20 μL) and iodine test solution (100 μL, 2.5 mM) and the absorbance of the solution was measured at 630 nm. The α-amylase inhibition was calculated and expressed as %. The 50% inhibitory concentration (IC50) of α-amylase was calculated through linear regression and the percentage of inhibition calculated according to the following equation:
| % inhibition = [1 − (Ab2 − Ab1)/(Ab4 − Ab3)] × 100, | (1) |
where Ab1 = absorbance of sample solution, Ab2 = absorbance of mixture without the enzyme, Ab3 = absorbance of mixture without the sample, and Ab4 = absorbance of mixture only starch without the sample and enzyme.
4.9.2. Determination of α-Glucosidase Inhibition
The α-glucosidase inhibitory activity was evaluated using the method by Kumar et al. [53]. Briefly, samples and standard stock solutions were prepared (10 mg/mL) and further diluted with 50 mM PBS (pH 6.9) to a final range of 0.5–5.0 mg/mL. The sample (50 μL) was mixed with α-glucosidase enzyme (50 μL, 0.57 unit/mL) dissolved in PBS (50 mM, pH 6.9) and then incubated in an orbital shaker (37 °C, 10 min). Then, 50 μL of substrate (15 mg of p-nitrophenyl α-d-glucopyranoside) was dissolved in 10 mL of PBS (50 mM, pH 6.9) and added to the reaction mixture. The reaction mixture was incubated again (37 °C, 20 min). The reaction was then stopped by adding 1 M Na2CO3 (50 μL) and the absorbance of the solution was measured at 405 nm. The α-glucosidase inhibition was calculated and expressed as %. The 50% inhibitory concentration (IC50) of α-glucosidase was calculated through linear regression and the percentage of inhibition calculated according to the following equation:
| % inhibition = [An − (As − Abs)/An)] × 100, | (2) |
where An = absorbance of negative solution (no sample), As = absorbance of sample solution, and Abs = absorbance of the blank sample solution.
4.10. Anti-Inflammatory Activity
4.10.1. Membrane Lysis Assay
Preparation of Erythrocyte Suspension
A suspension of erythrocytes was prepared according to the method described Shinde et al. [54], with little modifications. Briefly, human blood sample was collected from a healthy man. The blood was centrifuged (900 rcf, 5 min) and washed three times with an equal volume of normal saline NaCl (0.9% w/v). After centrifugation, the blood volume was measured and reconstituted (10% v/v) with an isotonic buffer solution (sodium phosphate buffer, 10 mM, pH 7.4).
Heat-Induced Hemolysis
The heat-induced hemolysis test was performed according to the method described by Okoli et al. [43] and Gunathilake et al. [55], with little modifications. Briefly, the blood cell suspension (0.05 mL) and the algal extract (0.05 mL) were mixed with PBS (2.95 mL, pH 7.4). The reaction mixture was incubated for 20 min at 54 °C. After incubation, the mixture was centrifuged at 2500 rcf for 3 min, and the absorbance of the supernatant measured at 540 nm. PBS was used as control. The level of hemolysis was calculated by using the following equation:
| % inhibition = (1 − As/Ac) × 100, | (3) |
where Ac = absorption of the control sample, and As = absorption of the sample.
Protein Denaturation Assay
Protein denaturation assay was performed according to the method described by Gambhire et al. [56], with little modifications. Briefly, the reaction mixture was prepared by adding 1% bovine serum albumin (BSA, 0.2 mL), PBS (4.78 mL, pH 6.4), and the algal extract (0.02 mL). The mixture was then incubated in a water bath (15 min, 37 °C), and the reaction mixture heated for 5 min at 70 °C. After cooling, the turbidity of the solution was measured at 660 nm. PBS was used as control. The percentage of inhibition of protein denaturation was calculated by using the following formula:
| % inhibition = (1 − As/Ac) × 100, |
where Ac = absorption of the control sample, and As = absorption of the sample.
Proteinase Inhibitory Activity
The proteinase inhibitory activity of the extracts was determined according to a method described by Sakat et al. [57], with little modification. Briefly, the reaction mixture was prepared by adding trypsin (0.06 mg), Tris-HCl buffer (1 mL, 20 mM, pH 7.4), and the algal sample (1 mL, containing 0.02 mL of algal extract and 0.980 mL of methanol). The reaction mixture was incubated for 5 min at 37 °C and then casein was added (1 mL, 0.8%, w/v). The reaction mixture was incubated again for additional 20 min at 37 °C and then 70% perchloric acid (2 mL) was added to stop the reaction. The reaction mixture was centrifuged at 2500 rcf for 5 min and the absorbance of the supernatant measured at 210 nm by using the buffer as blank. PBS was used as control. The percentage of inhibition of protein denaturation was calculated by the following formula:
| % inhibition = (1 − As/Ac) × 100, |
where Ac = absorption of the control sample, and As = absorption of the sample.
Lipoxygenase Inhibition Assay
The lipoxygenase activity of the extracts was evaluated as described by Wu et al. [58], with little modifications. Briefly, the reaction mixture containing sodium borate buffer (1 mL, 0.1 M, pH 8.8) and lipoxygenase (10 μL, final concentration 8000 U/mL) was incubated with 10 mL algal extract in a 1 mL cuvette at room temperature for 5 min (30 ± 2 °C). The reaction was initiated by the addition of linoleic acid (10 μL, 10 mmol). The absorbance of the reaction mixture was then measured at 234 nm. PBS was used as control, and the percentage of inhibition of the lipoxygenase activity was calculated by using the following equation:
| % inhibition = 100 × (Ac − As)/Ac, | (4) |
where Ac = absorbance of the control, As = absorbance of the sample.
4.11. Statistical Analysis
All experiments were carried out in triplicate and were expressed as mean ± standard deviation (SD). IC50 values were calculated by fitting the data through linear regression. The statistical significance between the obtained values (extract vs. standard) was assessed through a one way analysis of variance (ANOVA) (*, +, &, #, $ for p-values between 0.05 and 0.005; **, ++, &&, ##, $$ for p-values below 0.005). The correlation between TPC and TAC was estimated through linear regression and the resulting coefficient (R2) was used to establish the goodness of the fit. All the statistical analyses were performed by using GraphPad Prism 5 software (version 5.00, GraphPad Software Inc., La Jolla, CA).
5. Conclusions
Polyphenols present in the secondary metabolites from plant are gaining an even increasing attention because of their antioxidant effect. In this regard, marine algae represent a huge resource of secondary metabolites, although more studies are still needed to investigate their properties.
In this study secondary metabolites from Enteromorpha intestinalis were extracted by using three solvents with different polarity and physico-chemical characteristics, namely methanol, ethanol, and hexane. The extracts, especially that from the protic solvents, contained substantial amounts of phenols and flavonoids (up to about 60 and 625 mg of GAE/g and RUE/g, respectively, in the extract from ethanol), which were able to significantly reduce oxidative stress in vitro. The extract from ethanol was able to reduce hydrogen peroxide and superoxide anion radicals with IC50 values (about 3.2 and 2.7 μg/mL, respectively) almost comparable to those obtained from ascorbic acid (about 3.1 and 2.3 μg/mL, respectively). On the other hand, the extract from methanol yielded even better IC50 values in the DPPH• and hydroxyl radical scavenging assays (about 0.5 and 0.3 μg/mL, respectively) compared to AA (about 2.6 and 2.3 μg/mL, respectively). This antioxidant activity of the extracts also induced a potential antidiabetic efficacy, with the methanolic extract inhibiting α-amylase and α-glucosidase (IC50 of about 3.8 μg/mL in both cases) almost as well as acarbose (IC50 of about 2.8 and 3.3 μg/mL, respectively), a commercial antidiabetic drug. Similarly, the extract from methanol presented marked anti-inflammatory effect, as demonstrated by inhibition of hemolysis, protein denaturation, proteinase activity, and lipoxygenase activity, with IC50 values (about 10.8, 3.7, 5.8, and 4.7 μg/mL, respectively) almost as low as those obtained with acetylsalicylic acid (about 8.6, 3.1, 5.1, and 4.5 μg/mL, respectively).
With this strong antioxidant efficacy, the extracts, and in particular that from the alcoholic solution, could potentially represent valuable coadjuvants in the treatment of diabetes and other inflammatory diseases. However, additional studies are still required for isolating and identifying the many secondary metabolites responsible for the antioxidant effects, and for addressing their role in the protective action. Although this study represents just a preliminary starting point, the promising results obtained evidence the high potentiality of the secondary metabolites contained in the E. intestinalis. Future studies with a more detailed characterization of the extract, e.g., by NMR and HPLC, as well as of its biological effects, e.g., in cellular and animal studies, could eventually lead to the development of dietary supplements with, e.g., anti-aging activity, and/or to the discovery of novel drugs or prototypes with pharmacological effect toward oxidative stress-related diseases.
Acknowledgments
The authors are thankful to Berhampur University for providing the necessary facilities to carry out this work.
Author Contributions
B.P., R.N. and S.P. performed the experimental work. Writing—original draft preparation: B.P., C.B., R.N., and S.P.; visualization: B.P. and B.P.J.; review and editing: A.R. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by “Tecnopolo per la medicina di precisione” (TecnoMed Puglia)—Regione Puglia: DGR n.2117 del 21/11/2018, CUP: B84I18000540002 and “Tecnopolo di Nanotecnologia e Fotonica per la medicina di precisione” (TECNOMED)—FISR/MIUR-CNR: delibera CIPE n.3449 del 7-08-2017, CUP: B83B17000010001.
Data Availability Statement
Raw data are available upon request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tian C., Hao L., Yi W., Ding S., Xu F. Polyphenols, Oxidative Stress, and Metabolic Syndrome. Oxid. Med. Cell. Longev. 2020;2020:7398453. doi: 10.1155/2020/7398453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C., Rahu N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotas J., Leandro A., Monteiro P., Pacheco D., Figueirinha A., Gonçalves A.M.M., da Silva G.J., Pereira L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs. 2020;18:384. doi: 10.3390/md18080384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zafar M.S., Quarta A., Marradi M., Ragusa A. Recent developments in the reduction of oxidative stress through antioxidant polymeric formulations. Pharmaceutics. 2019;11:505. doi: 10.3390/pharmaceutics11100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 6.Mohanty S., Pradhan B., Patra S., Behera C., Nayak R., Jena M. Screening for nutritive bioactive compounds in some algal strains isolated from coastal Odisha. J. Adv. Plant Sci. 2020;10:1–8. [Google Scholar]
- 7.Farasat M., Khavari-Nejad R.A., Nabavi S.M., Namjooyan F. Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the Persian Gulf. Iran. J. Pharm. Res. 2014;13:163–170. [PMC free article] [PubMed] [Google Scholar]
- 8.Cho M., Kang I.J., Won M.H., Lee H.S., You S. The antioxidant properties of ethanol extracts and their solvent-partitioned fractions from various green seaweeds. J. Med. Food. 2010;13:1232–1239. doi: 10.1089/jmf.2010.1124. [DOI] [PubMed] [Google Scholar]
- 9.Pradhan B., Patra S., Behera C., Nayak R., Patil S., Bhutia S.K., Jena M. Enteromorpha compressa extract induces anticancer activity through apoptosis and autophagy in oral cancer. Mol. Biol. Rep. 2020;47:9567–9578. doi: 10.1007/s11033-020-06010-4. [DOI] [PubMed] [Google Scholar]
- 10.Wekre M.E., Kåsin K., Underhaug J., Holmelid B., Jordheim M. Quantification of polyphenols in seaweeds: A case study of Ulva intestinalis. Antioxidants. 2019;8:612. doi: 10.3390/antiox8120612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang H., Inoue M., Uzawa Y., Kawamura Y. Anti-tumorigenic components of a sea weed, Eeteromorpha clathrata. BioFactors. 2004;22:107–110. doi: 10.1002/biof.5520220121. [DOI] [PubMed] [Google Scholar]
- 12.Khanavi M., Gheidarloo R., Sadati N., Ardekani M.S., Nabavi S.M., Tavajohi S., Ostad S.N. Cytotoxicity of fucosterol containing fraction of marine algae against breast and colon carcinoma cell line. Pharmacogn. Mag. 2012;8:60–64. doi: 10.4103/0973-1296.93327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavoie S., Sweeney-Jones A.M., Mojib N., Dale B., Gagaring K., McNamara C.W., Quave C.L., Soapi K., Kubanek J. Antibacterial oligomeric polyphenols from the green alga Cladophora socialis. J. Org. Chem. 2019;84:5035–5045. doi: 10.1021/acs.joc.8b03218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan X., Yang C., Lin G., Chen Y., Miao S., Liu B., Zhao C. Antidiabetic potential of green seaweed Enteromorpha prolifera flavonoids regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. J. Food Sci. 2019;84:165–173. doi: 10.1111/1750-3841.14415. [DOI] [PubMed] [Google Scholar]
- 15.Lin G., Liu X., Yan X., Liu D., Yang C., Liu B., Huang Y., Zhao C. Role of green macroalgae Enteromorpha prolifera polyphenols in the modulation of gene expression and intestinal microflora profiles in type 2 diabetic mice. Int. J. Mol. Sci. 2018;20:25. doi: 10.3390/ijms20010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pradhan B., Patra S., Nayak R., Behera C., Dash S.R., Nayak S., Sahu B.B., Bhutia S.K., Jena M. Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: A journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 2020;164:4263–4278. doi: 10.1016/j.ijbiomac.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Pradhan B., Nayak R., Patra S., Jit B.P., Ragusa A., Jena M. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules. 2021;26:37. doi: 10.3390/molecules26010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrero-Miliani L., Nielsen O.H., Andersen P.S., Girardin S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace J.L., Vong L. NSAID-induced gastrointestinal damage and the design of GI-sparing NSAIDs. Curr. Opin. Investig. Drugs. 2008;9:1151–1156. [PubMed] [Google Scholar]
- 20.Maharana S., Pradhan B., Jena M., Misra M.K. Diversity of Phytoplankton in Chilika Lagoon, Odisha, India. Environ. Ecol. 2019;37:737–746. [Google Scholar]
- 21.Patra S., Pradhan B., Nayak R., Behera C., Rout L., Jena M., Efferth T., Bhutia S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2020:30215–30217. doi: 10.1016/j.semcancer.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan B., Patra S., Maharana S., Behera C., Dash S.R., Jena M. Demarcating antioxidant response against aluminum induced oxidative stress in Westiellopsis prolifica Janet 1941. Int. J. Phytoremediation. 2020:1–14. doi: 10.1080/15226514.2020.1807906. [DOI] [PubMed] [Google Scholar]
- 23.Ragusa A., Centonze C., Grasso M.E., Latronico M.F., Mastrangelo P.F., Fanizzi F.P., Maffia M. Composition and Statistical Analysis of Biophenols in Apulian Italian EVOOs. Foods. 2017;6:90. doi: 10.3390/foods6100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ragusa A., Centonze C., Grasso M.E., Latronico M.F., Mastrangelo P.F., Sparascio F., Fanizzi F.P., Maffia M. A Comparative Study of Phenols in Apulian Italian Wines. Foods. 2017;6:24. doi: 10.3390/foods6040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ragusa A., Centonze C., Grasso M.E., Latronico M.F., Mastrangelo P.F., Sparascio F., Maffia M. HPLC Analysis of Phenols in Negroamaro and Primitivo Red Wines from Salento. Foods. 2019;8:45. doi: 10.3390/foods8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulet J., Ducasse M.-A., Cheynier V. Ultraviolet spectroscopy study of phenolic substances and other major compounds in red wines: Relationship between astringency and the concentration of phenolic substances: UV spectroscopy of red wine components. Aust. J. Grape Wine Res. 2017;23:193–199. doi: 10.1111/ajgw.12265. [DOI] [Google Scholar]
- 27.Sudha G., Balasundaram A. Analysis of bioactive compounds in Padina pavonica using HPLC, UV-VIS and FTIR techniques. J. Pharmacogn. Phytochem. 2018;7:3192–3195. [Google Scholar]
- 28.Rajeswari R., Jeyaprakash K. Bioactive potential analysis of brown seaweed Sargassum wightii using UV-VIS and FT-IR. J. Drug Deliv. Ther. 2019;9:150–153. doi: 10.22270/jddt.v9i1.2199. [DOI] [Google Scholar]
- 29.Pradhan B., Patra S., Dash S.R., Nayak R., Behera C., Jena M. Evaluation of the anti-bacterial activity of methanolic extract of Chlorella vulgaris Beyerinck [Beijerinck] with special reference to antioxidant modulation. Futur. J. Pharm. Sci. 2021;7:17. doi: 10.1186/s43094-020-00172-5. [DOI] [Google Scholar]
- 30.Pradhan B., Patra S., Dash S.R., Maharana S., Behera C., Jena M. Antioxidant responses against aluminum metal stress in Geitlerinema amphibium. SN Appl. Sci. 2020;2:800. doi: 10.1007/s42452-020-2599-1. [DOI] [Google Scholar]
- 31.Crujeiras A.B., Díaz-Lagares A., Carreira M.C., Amil M., Casanueva F.F. Oxidative stress associated to dysfunctional adipose tissue: A potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic. Res. 2013;47:243–256. doi: 10.3109/10715762.2013.772604. [DOI] [PubMed] [Google Scholar]
- 32.Darley-usmar V.M., Hogg N., O’leary V.J., Wilson M.T., Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic. Res. Commun. 1992;17:9–20. doi: 10.3109/10715769209061085. [DOI] [PubMed] [Google Scholar]
- 33.Behera S.K. Phytochemical screening and antioxidant properties of methanolic extract of root of Asparagus racemosus Linn. Int. J. Food Prop. 2018;21:2681–2688. doi: 10.1080/10942912.2018.1560310. [DOI] [Google Scholar]
- 34.Aurand L.W., Boone N.H., Giddings G.G. Superoxide and singlet oxygen in milk lipid peroxidation. J. Dairy Sci. 1977;60:363–369. doi: 10.3168/jds.S0022-0302(77)83874-5. [DOI] [Google Scholar]
- 35.Pradhan B., Baral S., Patra S., Behera C., Nayak R., MubarakAli D., Jena M. Delineation of gamma irradiation (60Co) induced oxidative stress by decrypting antioxidants and biochemical responses of microalga, Chlorella sp. Biocatal. Agric. Biotechnol. 2020;25:101595. doi: 10.1016/j.bcab.2020.101595. [DOI] [Google Scholar]
- 36.Kedare S.B., Singh R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olokoba A.B., Obateru O.A., Olokoba L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012;27:269. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Coco L., Vergara D., De Matteis S., Mensà E., Sabbatinelli J., Prattichizzo F., Bonfigli A.R., Storci G., Bravaccini S., Pirini F., et al. NMR-Based Metabolomic Approach Tracks Potential Serum Biomarkers of Disease Progression in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2019;8:720. doi: 10.3390/jcm8050720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sudha P., Zinjarde S.S., Bhargava S.Y., Kumar A.R. Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement. Altern. Med. 2011;11:5. doi: 10.1186/1472-6882-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Standl E., Baumgartl H.J., Füchtenbusch M., Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes. Metab. 1999;1:215–220. doi: 10.1046/j.1463-1326.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- 41.Makinde E.A., Ovatlarnporn C., Sontimuang C., Herbette G., Olatunji O.J. Chemical Constituents from the Aerial Part of Tiliacora triandra (Colebr.) Diels and Their α-Glucosidase and α-Amylase Inhibitory Activity. Nat. Prod. Commun. 2020;15:1934578X19899595. doi: 10.1177/1934578X19899595. [DOI] [Google Scholar]
- 42.Umapathy E., Ndebia E.J., Meeme A., Adam B., Menziwa P., Nkeh-Chungag B.N., Iputo J.E. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J. Med. Plant Res. 2010;4:789–795. [Google Scholar]
- 43.Okoli C.O., Akah P.A., Onuoha N.J., Okoye T.C., Nwoye A.C., Nworu C.S. Acanthus montanus: An experimental evaluation of the antimicrobial, anti-inflammatory and immunological properties of a traditional remedy for furuncles. BMC Complement. Altern. Med. 2008;8:27. doi: 10.1186/1472-6882-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Govindappa M., Poojashri M.N. Antimicrobial, antioxidant and in vitro anti-inflammatory activity of ethanol extract and active phytochemical screening of Wedelia trilobata (L.) Hitchc. J. Pharmacogn. Phytother. 2011;3:43–51. [Google Scholar]
- 45.Rackova L., Oblozinsky M., Kostalova D., Kettmann V., Bezakova L. Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. J. Inflamm. 2007;4:15. doi: 10.1186/1476-9255-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khasawneh M.A., Elwy H.M., Hamza A.A., Fawzi N.M., Hassan A.H. Antioxidant, anti-lipoxygenase and cytotoxic activity of Leptadenia pyrotechnica (Forssk.) decne polyphenolic constituents. Molecules. 2011;16:7510–7521. doi: 10.3390/molecules16097510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rath J., Adhikary S.P. Algal Flora of Chilika Lake. Daya Publishing House; New Delhi, India: 2005. [Google Scholar]
- 48.Patra S., Panda P.K., Naik P.P., Panigrahi D.P., Praharaj P.P., Bhol C.S., Mahapatra K.K., Padhi P., Jena M., Patil S., et al. Terminalia bellirica extract induces anticancer activity through modulation of apoptosis and autophagy in oral squamous cell carcinoma. Food Chem. Toxicol. 2020;136:111073. doi: 10.1016/j.fct.2019.111073. [DOI] [PubMed] [Google Scholar]
- 49.Quettier-Deleu C., Gressier B., Vasseur J., Dine T., Brunet C., Luyckx M., Cazin M., Cazin J.C., Bailleul F., Trotin F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000;72:35–42. doi: 10.1016/S0378-8741(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 50.Al-Ani M., Opara L.U., Al-Bahri D., Al-Rahbi N. Spectrophotometric quantification of ascorbic acid contents of fruit and vegetables using the 2,4-dinitrophenylhydrazine method. J. Food Agric. Environ. 2007;5:165. [Google Scholar]
- 51.Patra S., Bhol C.S., Panigrahi D.P., Praharaj P.P., Pradhan B., Jena M., Bhutia S.K. Gamma irradiation promotes chemo-sensitization potential of gallic acid through attenuation of autophagic flux to trigger apoptosis in an NRF2 inactivation signalling pathway. Free Radic. Biol. Med. 2020;160:111–124. doi: 10.1016/j.freeradbiomed.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 52.Chakrabarti R., Singh B., Vanchhawng L., Thirumurugan K. Screening of nine herbal plants for in vitro α-amylase inhibition. Screening. 2014;7:84–89. [Google Scholar]
- 53.Kumar S., Kumar V., Prakash O. Enzymes inhibition and antidiabetic effect of isolated constituents from Dillenia indica. Biomed Res. Int. 2013;2013:382063. doi: 10.1155/2013/382063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shinde U.A., Phadke A.S., Nair A.M., Mungantiwar A.A., Dikshit V.J., Saraf M.N. Membrane stabilizing activity—A possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia. 1999;70:251–257. doi: 10.1016/S0367-326X(99)00030-1. [DOI] [Google Scholar]
- 55.Gunathilake K.D.P.P., Ranaweera K.K.D.S., Rupasinghe H.P.V. Influence of boiling, steaming and frying of selected leafy vegetables on the in vitro anti-inflammation associated biological activities. Plants. 2018;7:22. doi: 10.3390/plants7010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gambhire M., Juvekar A., Wankhede S. Evaluation of anti-inflammatory activity of methanol extract of Barleria cristata leaves by in vivo and in vitro methods. Int. J. Pharmacol. 2009;7:1–4. [Google Scholar]
- 57.Sakat S., Juvekar A.R., Gambhire M.N. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int. J. Pharm. Pharm. Sci. 2010;2:146–155. [Google Scholar]
- 58.Wu H. Affecting the activity of soybean lipoxygenase-1. J. Mol. Graph. 1996;14:331–337. doi: 10.1016/S0263-7855(97)00006-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data are available upon request from the authors.