Abstract
Background:
There is no study regarding the use of SOF/LDP in treatment of COVID-19.
Objectives:
In this study, the efficacy and safety of SOF/LDP were assessed in treatment of patients with mild to moderate COVID-19.
Methods:
Among an open-label randomized clinical trial, 82 patients with mild to moderated COVID-19 were assigned to receive either SOF/LDP 400/100 mg daily plus the standard of care (SOF/LDP group, n=42) or the standard of care alone (control group, n=40) for 10 days. Time to clinical response, rate of clinical response, duration of hospital and ICU stay and 14-day mortality were assessed.
Results:
Clinical response occurred in 91.46% of patients. Although rates of clinical response were comparable between the groups but it occurred faster in the SOF/LDP group than the control group (2 vs. 4 days respectively, P= 0.02). Supportive cares were provided in the medical wards for most patients but 17.07% of patients were transferred to ICU during the hospitalization course. However, durations of hospital and ICU stay were comparable between the groups. 14-day mortality rate was 7.14% and 7.5% in the SOF/ LDP and control groups respectively. No adverse effects leading to drug discontinuation occurred. Gastrointestinal events (nausea, vomiting and diarrhea) were the most common side effects (15.85%).
Conclusion:
Added to the standard of care, SOF/LDP accelerated time to the clinical response. However, rate of clinical response, duration of hospital and ICU stay and 14-day mortality were not different. No significant adverse event was detected. More randomized clinical trials with larger sample sizes are needed to confirm the efficacy and safety of SOF/LDP in the treatment of COVID-19. (www.actabiomedica.it)
Keywords: Sofosbuvir/ledipasvir, COVID-19, efficacy, safety
Introduction
A series of viral pneumonia emerged in December 2019 in Wuhan, China that was later named as coronavirus disease 2019 (COVID-19) caused by a novel coronavirus, SARS-CoV-2. (1-2) Patients mostly present with fever, cough, dyspnea, fatigue and myalgia with laboratory findings mostly consisting of lymphopenia and increased inflammatory biomarkers. (3) Diagnosis is based on clinical features, imaging studies (peripheral ground glass opacities in chest radiograph) and RT-PCR. (4) There is no approved drug for the treatment of COVID-19 and basically efforts should be made to manage patients with supportive care.
The immune response in COVID-19 happens in two phases i.e. viral phase in which the immune system helps eliminate the virus and cytokine release phase in which lung damage will progress. (5)
Coronaviruses are members of nidovirale order which consist of alfa, beta, gamma and delta family that have the largest known RNA.6 SARS-CoV-2 is a member of betacoronavirus family which also includes SARS-CoV and MERS-CoV strains, causes of outbreaks in 2002 and 2013. (6-8) Coronavirus genome is translated into two groups of proteins: structural and non-structural ones. (9) The genetic material also encodes 16 non-structural proteins (NSP); RNA-dependent RNA polymerase (RdRp) is one of the most important ones in virus replication. The life cycle of coronavirus is similar to other RNA viruses like hepatitis C virus (HCV) with similar enzymes and proteins needed for viral replication. (10)
Sofosbuvir/ ledipasvir (SOF/LDP) is an FDA approved drug for the treatment of HCV infection. (11) Sofosbuvir inhibits NS5B-RdRp which is a crucial enzyme in the replication of hepatitis C virus and ledipasvir inhibits NS5A which is an essential protein for RdRp function. (12)
Based on in vitro experiments with docking, SOF/LDP may be potential drugs for the treatment of COVID-19 but large clinical studies are required to confirm the idea (10, 13-15). In this study, efficacy and safety of SOF/LDP were assessed in treatment of patients with mild to moderate COVID-19.
Methods
This randomized open-label clinical trial was designed to evaluate efficacy and safety of SOF/ LDP in treatment of patients with mild to moderate COVID-19. Ethics committee of Tehran University of Medical Sciences, Tehran, Iran, approved the study (code number: IR.TUMS.VCR.REC.1398.1074). The trial was registered at Iranian Registry of Clinical Trials (IRCT: 20100228003449N29). Patients were admitted to Imam Khomeini Hospital Complex, a referral tertiary teaching hospital affiliated to Tehran University of Medical Sciences (TUMS), Tehran, Iran. Each patient provided an informed consent. Patients were divided into two groups (SOF/LDP or control group) based on the random allocation. Randomization was performed by permuted blocked randomization. To maintain allocation concealment, we used sequentially numbered, opaque sealed envelopes (SNOSE) method. Ninety identical letter-sized envelopes were provided. Numbers 1 to 90 were written on the envelopes; “SOF/LDP” was written on one set of 45, and “control” was written on the second set. Papers were folded and put in the envelopes. Based on randomized numbers, patients were enrolled in the groups.
Adult patients (≥18 years old) with highly suspected (according to the clinical signs/symptoms and imaging findings) or confirmed (a positive PCR of pharyngeal or nasopharyngeal samples) COVID-19 who were admitted to medical wards of the hospital, were included. Patients with history of drug allergy, decompensated cirrhosis, severe COVID-19, patients under hemodialysis and pregnant and lactating women were excluded. Mild and moderate COVID-19 were defined as (19):
Mild disease: mild symptoms such as fever, rhinorrhea, mild cough, sore throat, malaise, headache, muscle pain, or malaise, but with no shortness of breath AND no signs of a more serious lower airway disease AND RR <20, HR <90, oxygen saturation >93% on room air
Moderate disease: more significant lower respiratory symptoms, other than symptoms of mild disease including shortness of breath OR signs of moderate pneumonia, including RR≥ 20, oxygen saturation 88% on room air AND lung involvement less than 50 percent in chest X-ray or CT-scan.
Eligible patients received either SOF/ LDP (Zistdaru Danesh Co.) 400/90 mg daily for 10 days plus standard of care (SOF/ LDP group) or only standard of care (control group). In addition to the supportive care modalities, the standard of care according to the hospital protocol included hydroxychloroquine ( HCQ 400 mg BD at first day then 200 mg BD for 7 days) plus atazanavir/ritonavir 300/100 mg daily for 7 days.
Patients’ demographic characteristics, signs/symptoms, vital signs, baseline diseases and past drug history were recorded at the time of hospital admission. During the study period, patients were assessed daily regarding the clinical conditions and the disease progression. Laboratory and para-clinic findings were extracted from the hospital HIS. Patients were assessed daily for clinical response to therapy, time to the clinical response, adverse effects and complications during the hospitalization course.
All patients were followed for 14 days. Primary outcomes were clinical response and time to clinical response. Secondary outcomes were safety of treatment, complications during hospitalization, duration of hospital stay and 14-day mortality. Clinical response was defined as one order decline in disease category in the five category ordinal scale. The categories are: death (5), mechanical ventilation (4), non-invasive ventilation (3), oxygen mask or nasal cannula (2), discharge (1).
Estimating clinical response in 95% and 80% of patients in the SOF and control groups respectively, and with power of 80%, the sample size of the study was calculated as 80 persons.
All statistical analyses were performed using SPSS version 21 with independent sample t-test and Chi-square test. Normal distribution of variables was checked with Kolmogorov-Smirnov test. Continuous data are reported as median and interquartile range (IQR) and discrete data are reported as percentage. As continues variables had non-normal distribution, non-parametric test (Mann Whitney) was applied to compare these variables between the groups. P values less than 0.05 were considered statistically significant.
Kaplan-Meier survival was used to describe the possibility of hospital discharge and compared the groups using the log rank test. For bivariate analysis, Cox proportional hazards model adjusted for disease severity was applied.
Results
Of 116 patients who were assessed for eligibility, 90 met the inclusion criteria and underwent randomization. During study, 8 patients were excluded. Finally 40 patients in the control group and 42 patients in the SOF/ LDP group finished study (figure 1).
Figure 1.
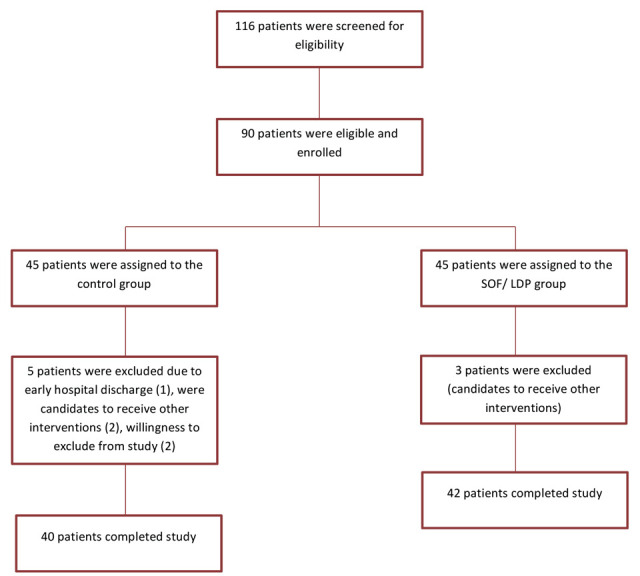
Consort flowchart of study
The median (IQR) age of the patients was 61.5 (45.5-74.25) and 63 (53.25-70.75) years in the SOF/ LDP and control group respectively. The most common baseline diseases were hypertension and diabetes mellitus (45.12%), cardiovascular diseases (31.70%) and hypothyroidism (10.97%). Angiotensin receptors blockers (37.80%) and metformin (32.93%) were common medications in patients’ drug history. Cough (64.63%), fever (56.1%), dyspnea (48.78%) were common symptoms in patients at the time of hospital admission. RT-PCR results were positive in 50% of patients. Lymphopenia (46.34%) and thrombocytopenia (23.17%) were common abnormal findings in complete blood count analysis. Most patients (87.80%) had some degree of lung involvements in the chest imagings. Median time from onset of the symptoms to hospital admission was 7 days. There was no significant difference between the groups regarding these baseline characteristics. (table 1)
Table 1.
Baseline characteristics of patients
| Character, median (IQR) or n(%) | SOF/ LDP group (n =42 ) | Control group (n =40) |
| Age (years) | 61.5 (46.5-74.25) | 63 (53.25-70.75) |
| Body mass index (Kg/m2) | 27 (25-30) | 25 (24-28) |
| Smoking | 1 (2.38%) | 2 (5%) |
| Baseline diseases | ||
| Diabetes mellitus | 22 (52.38%) | 15 (37.5%) |
| Hypertension | 20 (47.62%) | 17 (42.5%) |
| Cardiovascular diseases | 11 (26.19%) | 15 (37.5%) |
| Hypothyroidism | 5 (11.9%) | 4 (10%) |
| Rheumatoid arthritis | 2 (4.76%) | 2 (5%) |
| Chronic obstructive pulmonary disease | 3 (7.14%) | 1 (2.5%) |
| Malignancy | 1 (2.38%) | 2 (5%) |
| Transplant | 1 (2.38%) | 0 (0%) |
| Drug history | ||
| Angiotensin Π receptor blockers | 14 (33.33%) | 17 (42.5%) |
| Metformin | 16 (38.09%) | 11 (27.5%) |
| Beta blocker | 8 (19.05%) | 8 (20%) |
| Aspirin | 3 (7.14%) | 11 (27.5%) |
| Insulin | 5 (11.9%) | 7 (17.5%) |
| Hydroxychloroquine | 5 (11.9%) | 4 (10%) |
| Corticosteroids | 5 (11.9%) | 2 (5%) |
| Antibiotics | 4 (9.52%) | 3 (7.5%) |
| Vitamins and supplements | 3 (7.14%) | 9 (2.25%) |
| Non-steroidal anti-inflammatory drug | 3 (7.14%) | 0 (0) |
| Immunosuppressants | 2 (4.76%) | 1 (2.5%) |
| Angiotensin converting enzyme inhibitor | 2 (4.76%) | 1 (2.5%) |
| Methotrexate | 1 (2.38%) | 1 (2.5%) |
| Vital signs at the time of hospital admission | ||
| Temperature (°C) | 37.2 (36.77-37.9) | 37 (36.8-37.7) |
| Herat rate (beats/ min) | 94.5 (88-102.25) | 85 (78.25-97.5) |
| Respiratory rate (breaths/min) | 20 (19-22) | 20 (18.25-22) |
| Systolic blood pressure (mmHg) | 120 (110-130) | 120 (110-130) |
| O2 saturation (%) | 90 (88-93) | 90 (88-93.75) |
| Symptoms at the time of hospital admission | ||
| Cough | 31 (70.58%) | 22 (60%) |
| Fever | 21 (50%) | 25 (66.66%) |
| Dyspnea | 20 (47.05%) | 20 (43.33%) |
| Myalgia | 19 (41.17%) | 16 (43.33%) |
| Chills | 15 (35.29%) | 18 (50%) |
| Fatigue | 21 (38.23%) | 14 (36.66%) |
| Anorexia | 13 (32.35%) | 6 (16.66%) |
| Nausea | 11 (20.58%) | 7 (23.33%) |
| Vomiting | 9 (23.52%) | 7 (23.33%) |
| Chest discomfort | 9 (23.52%) | 5 (16.66%) |
| Headache | 10 (23.52%) | 4 (6.66%) |
| Abdominal pain | 5 (11.76%) | 5 (13.33%) |
| Diarrhea | 6 (11.76%) | 1 (3.33%) |
| Anosmia | 6 (14.70%) | 0 (0) |
| Positive RT-PCR | 22 (52.38%) | 19 (47.5%) |
| Laboratory data at the time of hospital admission | ||
| White Blood Cell (cells/mm3) | 6150 (4675-8425) | 5500 (4825-7500) |
| Lymphocyte count (cells/mm3) | 1020 (855-1413) | 1000 (690-1575) |
| Hemoglobin (g/dl) | 12.65 (11.97-14.3) | 13 (11.85-13.5) |
| Platelet count (cells×103/mm3) | 215 (162.25-294) | 198 (140-250.5) |
| Blood Urea Nitrogen (mg/dl) | 14 (10.75-18) | 13.5 (9-17.75) |
| Serum creatinine (mg/dl) | 1.0 (0.8-1.2) | 1.1 (0.9-1.4) |
| Sodium (meq/l) | 137.5 (135-140.25) | 138 (136-141) |
| Potassium (meq/l) | 4.45 (4-4.7) | 4.2 (3.9-4.57) |
| Calcium (mg/dl) | 8.5 (8-9) | 8.2 (7.97-8.75) |
| Phosphorus (mg/dl) | 3 (2.7-3.37) | 3.15 (2.9-3.7) |
| Magnesium (mg/dl) | 1.9 (1.8-2.2) | 2.1 (1.8-2.2) |
| Aspartate aminotransferase (U/l) | 31 (18.5-43.5) | 29 (20-38.25) |
| Alanine aminotransferase (U/L) | 26 (15.5-36.5) | 22 (14.75-36.75) |
| Alkaline phosphatase (U/l) | 171 (143-185) | 165.5 (113.75-220) |
| Bilirubin (mg/dl) | 0.5 (0.4-0.75) | 1 (0.6-1) |
| INR | 1 (1-1.1) | 1 (0.8-1.1) |
| Albumin (g/dl) | 3.85 (3.35-4.5) | 3.8 (3.3-3.95) |
| C-reactive protein (mg/dl) | 55 (41.25-129.75) | 64 (47-87) |
| Erythrocyte sedimentation rate (mm/h) | 73 (47.25-89.25) | 76 (51.5-89) |
| Lactate dehydrogenase (U/l) | 472 (393.5-636.5) | 540 (375.5-620.5) |
| Creatine phosphokinase (U/l) | 126 (34-205.5) | 125 (85-157) |
| Lymphopenia | 20 (47.62%) | 18 (45%) |
| Thrombocytopenia | 8 (19.05%) | 11 (27.5%) |
| Leukopenia | 4 (9.52%) | 5 (12.5%) |
| Leukocytosis | 2 (4.76%) | 3 (7.5%) |
| Hyponatremia | 8 (19.05%) | 5 (12.5%) |
| Hypokalemia | 1 (2.38%) | 2 (5%) |
| Hypomagnesimia | 18 (42.86%) | 11 (27.5%) |
| Hyperkalemia | 3 (7.14%) | 1 (2.5%) |
| Hypophosphatemia | 2 (4.76%) | 0 (0) |
| Chest radiograph involvement | 36 (85.71%) | 36 (90%) |
| Time from onset of the symptoms to hospital admission (day) | 7 (3.75-10) | 7 (4-10) |
| Stage of disease | ||
| Mild disease | 24 (57.14%) | 22 (55%) |
| Moderate disease | 18 (42.86%) | 18 (45%) |
One patient had contraindication (uncontrolled arrhythmia) to receive HCQ. Most patients received atazanavir/ritonavir (89.02%). Indications for starting stress ulcer and deep vein thrombosis prophylaxis were assessed for all patients and 95.12% and 92.68% of patients received an agent respectively. Antibiotics and corticosteroids were considered for 20.73% and 13.41% of patients respectively.
Median peripheral oxygen saturation in admitted patients was 90%. Most patients required respiratory support and received oxygen through face mask (68.29%), nasal cannula (14.63%), invasive (8.53%) or non-invasive (2.44%) ventilation. There was no significant difference between the groups regarding concomitant medications. (table 2)
Table 2.
Medications and supportive cares
| Variable | SOF/ LDP group (n = 42) | Control group (n = 40) | P value |
| Hydroxychloroquine | 42 (100%) | 39 (97.5%) | 0.49 |
| Atazanavir | 39 (92.85%) | 34 (85%) | 0.22 |
| Proton pump inhibitor | 37 (88.09%) | 34 (85%) | 0.46 |
| Heparin | 39 (92.85%) | 33 (82.5%) | 0.14 |
| Diphenhydramine | 18 (42.86%) | 14 (35%) | 0.31 |
| Atorvastatin/ rosuvastatin | 6 (14.28%) | 17 (42.5%) | 0.004 |
| Naproxen | 9 (21.43%) | 14 (35%) | 0.13 |
| Angiotensin Π receptor blockers | 9 (21.43%) | 11 (27.5%) | 0.35 |
| Antibiotics | 11 (26.19%) | 8 (20%) | 0.34 |
| Corticosteroids | 7 (16.66%) | 4 (10%) | 0.29 |
| Beta blocker | 7 (16.66%) | 4 (10%) | 0.29 |
| Antiemetics | 2 (4.76%) | 5 (12.5%) | 0.19 |
| H2 receptor blocker | 3 (7.14%) | 4 (10%) | 0.47 |
| Low molecular weight heparin | 2 (47.62%) | 2 (5%) | 0.67 |
| Vitamin C | 4 (9.52%) | 0 (0) | 0.06 |
| Angiotensin converting enzyme inhibitor | 1 (2.38%) | 2 (5%) | 0.48 |
| Respiratory supports | |||
| Nasal cannula | 3 (7.14%) | 9 (22.5%) | 0.53 |
| Face mask | 34 (80.95%) | 22 (55%) | |
| Non-invasive ventilation | 2 (4.76%) | 0 (0) | |
| Mechanical ventilation | 3 (7.14%) | 4 (10%) | |
| Site of care | |||
| General ward | 35 (83.33%) | 33 (82.5%) | 0.58 |
| Intensive care unit | 7 (16.66%) | 7 (17.5%) | |
Table 3.
Outcomes and adverse effects
| Variable | SOF/ LDP group (n = 42) | Control group (n = 40) | P value |
| Clinical response | 38 (90.48%) | 37 (92.5%) | 0.65 |
| Time to clinical response (day) | 2 (1-3.75) | 4 (2-5) | 0.02 |
| Duration of hospital stay (day) | 4 (2-9.5) | 5 (3.25-7) | 0.98 |
| Duration of ICU stay (day) | 6 (4-11) | 9 (6-12) | 0.23 |
| 14-day mortality | 3 (8.82%) | 3 (10%) | 0.60 |
| Drug adverse events | |||
| Cardiovascular | 2 (4.76%) | 3 (7.5%) | 0.48 |
| Kidney injury | 3 (7.14%) | 0 (0) | 0.08 |
| Hyperbilirubinemia | 3 (7.14%) | 0 (0%) | 0.33 |
| Gastrointestinal | 6 (14.28%) | 7 (17.5%) | 0.46 |
| Headache | 1 (2.38%) | 1 (2.5%) | 0.74 |
Clinical response occurred in 91.46% of patients. Although rates of clinical response were comparable between the groups but response occurred faster in the SOF/LDP group than the control group (2 vs. 4 days respectively, P= 0.02). The possibility of discharge in the two groups is shown in Kaplan-Meier plot (Fig 2). The analysis showed no significant difference between the groups (95% CI:3.45-5.19, p=0.17)
Figure 2.
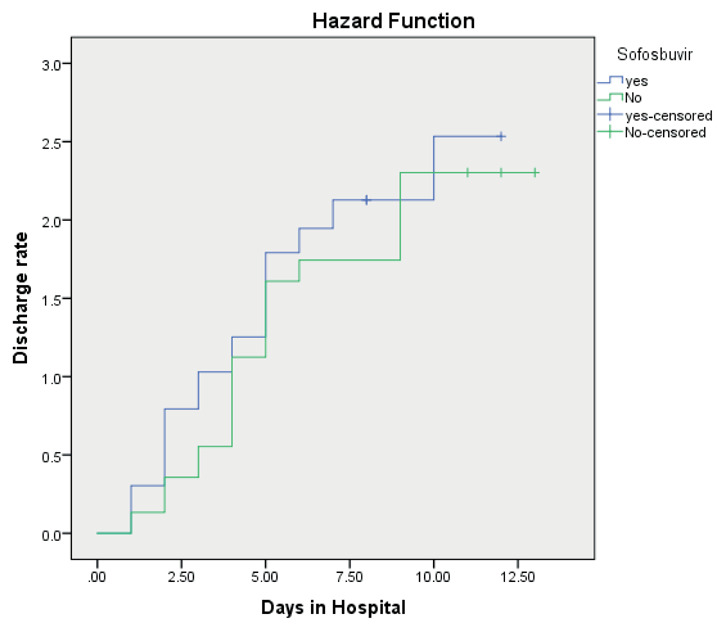
Kaplan-Meier curves for the possibility of discharge from hospital
Use of SOF/LDP was associated with statistically insignificant shortened length of hospital stay when adjusted for disease severity (HR: 1.55, 95% CI: 0.97-2.49, p=0.06).
Supportive cares were provided in the medical wards for most patients but 17.07% of patients were transferred to ICU during the hospitalization course. Durations of hospital and ICU stay were comparable between the groups. 14-day mortality rate was 7.14% and 7.5% in the SOF/ LDP and control groups respectively.
No adverse effects leading to drug discontinuation occurred. Gastrointestinal events (nausea, vomiting and diarrhea) were the most common side effects (15.85%). QTc prolongation (more than 60 miliseconds) was detected in 6.09% of patients. Acute kidney injury and hyperbilirubinemia occurred in 3 patients in the SOF/ LDP group during the course of hospitalization. Two out of 3 patients had septic shock. After recovery from the shock, serum creatinine and bilirubin returned near to the normal range.
Discussion
In this trial, efficacy and safety of SOF/LDP in the treatment of the new emerging viral pneumonia, COVID-19 was assessed. Comparing with the standard of care, adding SOF/LDP did not change rate of clinical response, length of hospital or ICU stay and 14-day mortality in patients with mild to moderate COVID-19 pneumonia. However, time to clinical response was significantly shorter in the SOF/ LDP than the control group. Adverse effects were also comparable between the groups.
Similar with previous reports, cough, fever and dyspnea were common chief complaints at the time of hospital admission. Also lymphopenia was the most common laboratory abnormality. (3,20)
There are two randomized and one non-randomized clinical trials evaluating the effect of sofosbuvir/ daclatasvir (FOS/DCV) in hospitalized patients with COVID-19. A study by Sadeghi et al. (16) included 66 patients with severe disease that received either FOS/DCV daily plus hydroxychloroquine (with or without lopinavir/ritonavir;LPN/r) or hydroxychloroquine (with or without LPN/r) for 14 days. 33% and 67% of the patients in SOF/DCV and control group received LPN/r (P=0.026). The primary outcome of the study was clinical recovery within 14 days with met in 88% and 67% of SOF/DCV and control groups respectively (P=0.076); duration of hospitalization was also significantly shorter in SOF/DCV group (P=0.041). Clinical recovery in SOF/DCV was observed in 82% of patients treated with LPN/r compared to 91% who didn’t receive the combination. All-cause mortality and need for mechanical ventilation were comparable between the groups. (P=0.708, P=0.303 respectively). Patients in both groups received same concomitant corticosteroids (36% vs 24%; P=0.422).
Kasgari et al. (17) studied the effect of SOF/DCV in 48 hospitalized patients with mild to moderate COVID-19. 24 patients were treated with SOF/DCV plus ribavirin and the other 24 received hydroxychloroquine plus LPN/r with or without ribavirin. Time from symptom onset to hospitalization was similar between the groups. Median time to recovery and duration of hospitalization was 6 days in both groups. The overall mortality was 6%, all observed in the control group with younger patients and higher rate of diabetes (P=0.006). Four patients in the control group needed ICU admissions and mechanical ventilation but the difference with SOF/DCV group was not statistically significant (P=0.109). In this study, ribavirin was used in the intervention group and it is not known whether there was any synergistic effect with SOF/DCV and ribavirin concomitant use.
Another study conducted by Eslami et al. (18) enrolled 62 patients with severe COVID-19 to receive either SOF/DCV (35 patients) or ribavirin (27 patients) for 14 days both with single dose hydroxychloroquine and LPN/r (for 5 days). Median duration of hospital stay (5 vs 9; P<0.01), time to recovery (6 vs 11; P<0.01), ICU admissions (6 vs 13; P=0.01) and rate of death (2 vs 9; P=0.01) were significantly different between the groups.
These three clinical trials show that sofosbuvir/ daclatasvir could reduce the time to recovery in COVID-19 patients but the efficacy of sofosbuvir alone should also be evaluated. Also the study population in these trials was small and larger blinded randomized clinical trials are required to draw a definite result (21). Patients with different severity of the disease were included and some concomitant treatment also were applied.
Several other agents were examined or are under investigation for treatment of COVID-19. During a randomized clinical trial, lopinavir/ ritonavir did not significantly change time to clinical improvement, 28-day mortality and viral RNA load in patients with severe COVID-19. Median time to clinical improvement was 16 days for patients who received lopinavir/ritonavir or the standard of care. Patients who received lopinavir/ ritonavir experienced more gastrointestinal adverse effects. (22)
HCQ was another agent which was examined in the treatment of mild to moderate COVID-19. In this study, HCQ (loading dose of 1200 mg daily for three days followed by a maintenance dose of 800 mg daily till 2 weeks) was compared with the standard care. HCQ showed no added benefit to the standard care for conversion of SARS-CoV-2. Time to negative conversion was 7 and 8 days in the control and HCQ group respectively. More gastrointestinal adverse effects were detected in the HCQ group than the control group.(23)
Although adding azithromycin to HCQ increased the rate of viral clearance, but clinical response was not evaluated. (24)
In data analysis of 96032 hospitalized COVID-19 patients (a multi-center study from 6 countries), efficacy and safety of chloroquine, chloroquine plus a macrolid (azithromycin or clarithromycin), hydroxychloroquine, hydroxychloroquine plus a macrolid and control group were evaluated. Comparing with the control group, all medications increased mortality and ventricular arrhythmia. Length of hospital stay and rate of mechanical ventilation were also higher in the treatment groups. No benefit was detected with chloroquine, hydroxychloroquine or macrolids in hospitalized patients with COVID-19. (25)
In-hospital mortality and cardiac adverse effects of hydroxychloroquine and azithromycin (alone or in combination) were assessed in COVID-19 patients. No statistically significant difference in mortality was detected. Patients received HCQ plus azithromycin experienced more cardiac arrests. (26)
Efficacy and safety of remdesivir, an inhibitor of virus RNA polymerase, were evaluated in hospitalized patients with severe COVID-19. Patients who received remdesivir (200 mg on day 1 followed by 100 mg on days 2–10) numerically (but not statistically significant) experienced faster time to clinical improvement than those who received the placebo (21 days vs 23 days). 28-day mortality and incidence of adverse effects were comparable between the groups. (27)
Previous findings were repeated in Beigel et al study. Time to clinical recovery in remdesivir group was shorter compared with the placebo group (11 days vs. 15 days). Also 14-day mortality was not different between the groups. (28) Goldman et al did not find any significant difference between 5-day and 10-day remdesivir treatment course in patients with severe COVID-19. Patients in the 10-day group had more severe disease and a little longer time to clinical recovery compared with patients in the 5-day group. This trial didn’t include placebo group and didn’t assess the efficacy of remdesivir. (29)
Efficacy of favipiravir (1600 mg BID day 1 and 600mg BID till day 14) as add-on therapy was compared with lopinavir/ ritonavir (400/100mg for 14 days) in patients receiving IFN-a by aerosol inhalation. Patients in favipiravir group experienced shorter time to viral clearance (4 days vs 11 days) and more improvement in chest imaging in 14 days (91.43% vs 62.22%) compared with the lopinavir/ ritonavir group. (30)
Effectiveness of interferons in treatment of COVID-19 has been reported. Nebulized interferon a-2b (with or without arbidol) may help recovery from COVID-19 by reducing load of viral inflammatory markers. (31) Combination of interferon beta-1b, lopinavir/ritonavir and ribavirin in hospitalized patients with mild to moderate disease decreased duration of hospital stay and viral load compared to lopinavir/ritonavir alone. (32)
Similar with other studies (33-34) hypertension and diabetes mellitus were the most common baseline diseases in COVID-19 patients. More patients in the control group were receiving statins before diagnosis of COVID-19 than in the SOF/ LDP group. Statins are well known for their anti-inflammatory and antioxidant properties. There are no RCTs to investigate the effects of statins in COVID-19 but in some other viral infections like H1N1 influenza (35) and Ebola (36) statins decreased disease severity. Statins also up-regulate ACE2 that is the tissue receptor for coronavirus. (37, 38) It was hypothesized that higher levels of ACE2 receptor can decrease the severity of lung injury in COVID-19. (39) Patients with COVID-19 are susceptible to cardiovascular events and statins may protect the patients by preventing cardiovascular damage. (40)
Four patients in the SOF/LDP group received low dose (1000 mg daily) of parenteral vitamin C. Vitamin C has antioxidant properties but its efficacy in the treatment of COVID-19 has not been defined. There is an ongoing trial to assess effects of high dose vitamin C on mortality and length of hospital stay in patients with COVID-19. (41)
SARS-CoV-2 is in a member of nidoviridale order and betacoronaviridae family. Four viruses in this family are known as causes of common cold (pharyngitis, rhinitis, sinusitis, diarrhea). SARS and MERS-CoV are two other members that caused fatal pneumonia outbreaks in 2003 and 2014 respectively.5-7 The seventh strain of the family is SARS-CoV-2 that has the genetic material 80% identical to SARS-CoV and 50% identical to MERS-CoV.33 SARS-CoV-2 genome is an enveloped, single-stranded, positive-sense RNA with the first 2/3 of the genome translated into 16 non-structural proteins (NSPs) and the other 1/3 translated into non-structural proteins (spike, envelope, nucleocapsid, membrane proteins). (42) RNA-dependent RNA polymerase (RdRp) is one of the most important NSPs and is a crucial viral enzyme in the life cycle of different viruses, including the hepatitis C virus (HCV), the Zika virus (ZIKV), and coronaviruses (CoVs). (43-45)
Sofosbuvir is a direct-acting antiviral with NS5B-RdRp inhibitory property. (46) In 2014, the combination of SOF/LSD (a NS5A protein inhibitor) received US FDA approval for the treatment of genotype 1 HCV patients. HCV NS5B-RdRp requires nucleotide triphosphates as substrate to synthesize RNA, a process with no proofreading function. Sofosbuvir acts like the substrate of the enzyme which terminates the RNA synthesis by incorporating into the RNA chain. (47)
The two-phase immune response i.e. viral and cytokine release happens in COVID-19. Treatment strategies for COVID-19 are categorized into inhibition of CoV fusion/entry, disruption of CoV replication and suppression of excessive inflammatory response.5 If patient enters the cytokine release state then the antiviral medications do not seem to help resolving the disease. Regarding the phase of the disease and the pathway targeted for treatment, distinct approaches are helpful.
There are in silico studies which assessed the potential activity of sofosbuvir against coronavirus RdRp. Sofosbuvir successfully bonds with the coronavirus polymerase.13In two studies published in 2020, sofosbuvir inhibited coronavirus replication by fitting into enzyme active site.(14-15) Ledipasvir has ability to attach to main protease enzymes of the novel coronavirus and in combination with sofosbuvir, it may be a candidate for the treatment of COVID-19. (10)
There is no data about the 50% of maximum inhibitory concentration (IC 50) of sofosbuvir for coronavirus. However, different values of IC were reported for hepatitis C, hepatitis E, hepatitis A, ZIKA, Dengue and West Nile virus. For HCV it was detected between 0.016– 1.2 µM. (48, 49) According to the replicons, sofosbuvir inhibited HEV replication with IC 50 between 1.2-10 µM.46 Dose-dependent activity of sofosbuvir against HEV has also been reported. (51, 52)
In-vitro activity (IC 50 of 6.3µΜ) of sofosbuvir against hepatitis A virus was detected. (53) The IC 50 of 0.38-44µM was reported for 3 strains of Zika virus. (54-57) In term of West Nile virus (WNV), the IC 50 values of sofosbuvir were 1.2μM and 63.4μM in liver and lung cells respectively. (58) Compared with hepatic cells, concentrations of sofosbuvir were much lower in kidney and lung cells. It could be due to sofosbuvir being less activated in these cells. (47) Other studies on different cell lines are needed to evaluate the activity of sofosbuvir in tissues other than hepatic cells.
Data regarding effectiveness of antivirals against SARS-CoV2 are scarce. It seems that if antiviral medications are considered, they should be initiated as soon as possible in early stage of the infection, when the damage to lung tissues has not extended. When the inflammatory process starts and cytokine storm takes place, no benefit is expected to be seen with antiviral drugs. Sofosbuvir is an available drug in many countries and can play a role in the treatment of mild to moderate COVID-19. However, clinical trials with larger sample sizes are needed to confirm efficacy of sofosbuvir in treatment of COVID-19.
Due to the special circumstances and multiplicity of the clinical trials, we could not enroll more patients in our study to draw a definite conclusion. Also considering follow-up RT-PCR and chest imaging were not possible.
Conclusion
To the best of our knowledge, this was the first study that evaluated efficacy and safety of SOF/LDP in the treatment of mild to moderate COVID-19. Added to the standard of care, SOF/LDP accelerated time to the clinical response. However, rate of clinical response, duration of hospital or ICU stay and 14-day mortality were not different. No significant adverse event was detected. More randomized clinical trials with larger sample sizes are needed to confirm efficacy and safety of SOF/LDP in the treatment of COVID-19.
Acknowledgement
We would like to thank the nurses and other staffs of Imam Khomeini Hospital Complex for their kind supports.
Funding:
The authors did not receive any fund for this work. SOF/LDP was a generous donation from Zistdaru Danesh Co.
Declaration of interest:
There is no conflict of interest for authors to declare.
References
- 1.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China. N Eng J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F.-W, Yuan S, Kok K.-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y-H, Cai L, Cheng Z-S, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1953–66. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 8.Zaki AM, Van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–20. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 9.Hilgenfeld R, Peiris M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antiviral Res. 2013;100(1):286–95. doi: 10.1016/j.antiviral.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YW, Yiu C-PB, Wong K-Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL pro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000 Research. 2020;9:129. doi: 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schinazi RF, Shi J, Whitaker T. Sofosbuvir (Sovaldi): The First-in-Class HCV NS5B Nucleotide Polymerase Inhibitor. Innovative Drug Synthesis. 2016:61–80. [Google Scholar]
- 12.Zuccaro V, Lombardi A, Asperges E, et al. PK/PD and antiviral activity of anti-HCV therapy: is there still a role in the choice of treatment? Expert Opin Drug Metab Toxicol. 2020;16(2):97–101. doi: 10.1080/17425255.2020.1721459. [DOI] [PubMed] [Google Scholar]
- 13.Elfiky AA, Mahdy SM, Elshemey WM. Quantitative structure-activity relationship and molecular docking revealed a potency of anti-hepatitis C virus drugs against human corona viruses. J Med Virol. 2017;89(6):1040–1047. doi: 10.1002/jmv.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadeghi A, Asgari AA, Norouzi A, et al. Sofosbuvir and daclatasvir compared with standard of care in the treatment of patients admitted to hospital with moderate or severe coronavirus infection (COVID-19): a randomized controlled trial. J Antimicrob Chemother. 2020 Aug 19 doi: 10.1093/jac/dkaa334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasgari HA, Moradi S, Shabani AM, et al. Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a singlecentre, randomized controlled trial. J Antimicrob Chemother. 2020 Nov 1;75(11):3373–3378. doi: 10.1093/jac/dkaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eslami G, Mousaviasl S, Radmanesh E, et al. The impact of sofosbuvir/ daclatasvir or ribavirin in patients with severe COVID-19. J Antimicrob Chemother. 2020 Aug 19 doi: 10.1093/jac/dkaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;24 doi: 10.1001/jama.2020.2648. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 20.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmons B, Wentzel H, Mobarak S, et al. Sofosbuvir/daclatasvir regimens for the treatment of COVID-19: an individual patient data meta-analysis. J Antimicrob Chemother. 2020 Oct 16 doi: 10.1093/jac/dkaa418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang W, Cao Z, Han M, Wang Z, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra MR, Desai SS, Ruschitzka F, et al. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020 doi: 10.1001/jama.2020.8630. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19—Preliminary Report. N Engl J Med. 2020 May 22 doi: 10.1056/NEJMc2022236. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 29.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 May 27 doi: 10.1056/NEJMoa2015301. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020 Mar 18 doi: 10.1016/j.eng.2020.03.007. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Q, Wei X-S, Xiang X, et al. Interferon-a2b treatment for COVID-19. medRxiv. 2020 [Google Scholar]
- 32.Hung IF-N, Lung K-C, Tso EY-K, et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020 May 30;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, Yu T, Du R, Fan G, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13 doi: 10.1001/jamainternmed.2020.0994. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99(3):417–435. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Fedson DS. A practical treatment for patients with Ebola virus disease. J Infect Dis. 2015;211(4):661–662. doi: 10.1093/infdis/jiu474. [DOI] [PubMed] [Google Scholar]
- 37.Lu R, Zhao X, Li J, Niu P, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tikoo K, Patel G, Kumar S, et al. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: Role of epigenetic histone modifications. Biochem Pharmacol. 2015;93(3):343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Fedson DS, Opal SM, Rordam OM. Hiding in plain sight: an approach to treating patients with severe COVID-19 infection. mBio. 2020;11(2):e00398–20. doi: 10.1128/mBio.00398-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1286. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 41.Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24:133. doi: 10.1186/s13054-020-02851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prajapat M, Sarma P, Shekhar N, et al. Drug targets for corona virus: A systematic review. Indian journal of pharmacology. Indian J Pharmacol. 2020;52(1):56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dustin LB, Bartolini B, Capobianchi MR, et al. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22(10):826–832. doi: 10.1016/j.cmi.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sirohi D, Kuhn RJ. Zika virus structure, maturation, and receptors. J Infect Dis. 2017;216(suppl_10):S935–S944. doi: 10.1093/infdis/jix515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fehr AR, Perlman S. Coronaviruses: An Overview of Their Replication and Pathogenesis. Coronaviruses. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babusis D, Curry MP, Kirby B, et al. Sofosbuvir and ribavirin liver pharmacokinetics in patients infected with hepatitis C virus. Antimicrob Agents Chemother. 2018;62(5):e02587–17. doi: 10.1128/AAC.02587-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pockros PJ. Nucleoside/nucleotide analogue polymerase inhibitors in development. Clin Liver Dis. 2013;17(1):105–10. doi: 10.1016/j.cld.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Lam AM, Espiritu C, Bansal S, et al. Genotype and subtype profiling of PSI-7977 as a nucleotide inhibitor of hepatitis C virus. Antimicrob Agents Chemother. 2012;56(6):3359–68. doi: 10.1128/AAC.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fenaux M, Lin X, Yokokawa F, et al. Antiviral nucleotide incorporation by recombinant human mitochondrial RNA polymerase is predictive of increased in vivo mitochondrial toxicity risk. Antimicrob Agents Chemother. 2016;60(12):7077–7085. doi: 10.1128/AAC.01253-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thi VLD, Debing Y, Wu X, et al. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology. 2016;150(1):82–85.e4. doi: 10.1053/j.gastro.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Netzler NE, Tuipulotu DE, Vasudevan SG, et al. Antiviral candidates for treating hepatitis E virus infection. Antimicrob Agents Chemother. 2019;63(6):e00003–19. doi: 10.1128/AAC.00003-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W, Hakim MS, Nair VP, et al. Distinct antiviral potency of sofosbuvir against hepatitis C and E viruses. Gastroenterology. 2016;151(6):1251–1253. doi: 10.1053/j.gastro.2016.09.061. [DOI] [PubMed] [Google Scholar]
- 53.Jiang W, Muhammad F, Ma P, et al. Sofosbuvir inhibits hepatitis A virus replication in vitro assessed by a cell-based fluorescent reporter system. Antiviral Res. 2018;154:51–57. doi: 10.1016/j.antiviral.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Bullard-Feibelman KM, Govero J, et al. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antiviral Res. 2017;137:134–140. doi: 10.1016/j.antiviral.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vicenti I, Dragoni F, Giannini A, et al. Development of a Cell-Based Immunodetection Assay for Simultaneous Screening of Antiviral Compounds Inhibiting Zika and Dengue Virus Replication. SLAS Discov. 2020;25(5):506–514. doi: 10.1177/2472555220911456. [DOI] [PubMed] [Google Scholar]
- 56.Sacramento CQ, de Melo GR, Rocha N, et al. The clinically approved antiviral drug sofosbuvir impairs Brazilian zika virus replication. bioRxiv. 2016 061671. [Google Scholar]
- 57.Mumtaz N, Jimmerson LC, Bushman LR, et al. Cell-line dependent antiviral activity of sofosbuvir against Zika virus. Antiviral Res. 2017;146:161–163. doi: 10.1016/j.antiviral.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Dragoni F, Boccuto A, Picarazzi F, et al. Evaluation of sofosbuvir activity and resistance profile against West Nile virus in vitro. Antiviral Res. 2020;175:104708. doi: 10.1016/j.antiviral.2020.104708. [DOI] [PubMed] [Google Scholar]


