Abstract
Background
The sudden outbreak of the COVID-19 disease originated in Wuhan, China, in December 2019. There have been few reports on the clinical course of the disease, but detailed information on the risk factors for increased hospital stay and mortality is not available. In this study we aimed to present the details of 53 confirmed COVID-19 cases to share the clinical course and the risk factors for longer hospital stay and death.
Methods
In this study, we enrolled fifty-three patients with confirmed COVID-19 infection from a referral academic hospital in Tehran, Iran admitted between March and April 2020. Patients’ demographics, laboratory tests, treatments, length of hospital stay (LOHS), and final outcome were recorded and analyzed.
Results
Fifty-three patients were included in this study. The higher LOHS was associated with clinical symptoms, including hemoptysis (IRR= 0.73, P-value= 0.02), diarrhea (IRR= 0.78, P-value= 0.01), headache (IRR= 0.81, P-value= 0.05), and dry cough (IRR= 0.82, P-value= 0.05). Mortality was associated with older age (Odds ratio=1.148, 95%CI=1.032-1.276), lower calcium level (Odds ratio=0.087, 95%CI=0.010-0.788), lower serum albumin (Odds ratio=0.036, 95%CI=0.002-0.655), as well as increased level of neutrophil/lymphocyte ratio (NLR) (Odds ratio=1.468, 95%CI=1.086-1.985), lactate dehydrogenase (LDH) (Odds ratio=1.004, 95%CI=1.000-1.007), and urea (Odds ratio=1.023, 95%CI=1.006-1.039).
Conclusion
Our study identified that decreased levels of O2 saturation, platelet count, calcium, albumin, and increased NLR, LDH, urea and old age were correlated with mortality. Also, LOHS was significantly associated with clinical findings, such as hemoptysis and diarrhea. (www.actabiomedica.it)
Keywords: COVID-19, SARS-CoV-2, Mortality, Hospitalization, Risk factors
Introduction
On December 2019, an outbreak emerged in Wuhan, China which caused a cluster of cases with a viral pneumonia[1]. Soon after, the disease rapidly spread around the world with later declaration of pandemic by World Health Organization (WHO)[2, 3]. Intensive research and High-throughput sequencing confirmed that a novel coronavirus, SARS-CoV-2, was responsible for this cluster[4].
SARS-CoV-2 is a member of Coronaviridae family, enveloped-RNA viruses, that infects mammalian and bird cells and could stimulate the respiratory and digestive systems[5]. SARS-CoV-2 has a higher rate of spread than its counterparts, SARS-CoV and MERS-CoV and can be transmitted person-to-person in hospital and family setting[6]. The transmission is via contaminated droplets and fomites during unprotected close contact. The patients commonly present with fever, cough, dyspnea, rhinorrhea, headache, myalgia and arthralgia. However, there is no characteristic clinical hallmark for the coronavirus disease (COVID-19) and clinical course may vary from no symptoms (asymptomatic cases) to severe pneumonia and death[7]. Most patients have mild to moderate disease, while some cases need critical care. According to the current data, high risk individuals are often the ones with underlying diseases and those who are over 60 years old[8]. Although the cases of the COVID-19 is increasing rapidly worldwide, a large number of cases recover after hospitalization and low mortality rates have been reported[9, 10].
Despite the current epidemiologic data, the clinical features of the COVID-19 are still not elucidated[1, 11, 12]. Further global investigations are required to illustrate the clinical characteristics of the COVID-19 infection.
Here we present the details of 53 laboratory confirmed cases with definite outcomes (death or discharge), admitted to a referral center, to share the clinical course and the risk factors for longer hospital stay and death.
Material and Method
Study design and patients
This retrospective cohort study was conducted on adults >18 years old who were admitted to Shahid Labbafi Nejad Hospital, Tehran, Iran. The study was approved by the institutional ethics committee and written informed consent was obtained from all participants.
Fifty-three patients with definite diagnosis of COVID-19 who were admitted to our hospital between 1 March 2020 and 10 April 2020 were enrolled in this study. The individuals were initially screened for the hospitalization criteria according to their clinical presentation, including SPO2˂93%, acute respiratory distress, impaired general condition, or respiratory rate˃30 per minute. Patients with any of the aforementioned conditions were hospitalized. Then, the diagnosis was confirmed by reverse transcriptase polymerase chain reaction (RT-PCR) test. Epidemiological, demographic, clinical manifestations, past medical history, laboratory, medication, and outcome data were recorded.
Measurements
A descriptive questionnaire designed by the Shahid Labbafi Nejad Educational and Medical Center was used to record the possible risk factors of the patients, containing information about travel history in the risk period, onset date of symptoms, underlying comorbidities, history of influenza vaccination, hospitalization and treatment details. Laboratory tests, including complete blood count (normal range: WBC=4000-11000) and differentiation, blood chemistry, coagulation test (normal range: PT=10-12, PTT=25-35), electrolytes (normal range: Na=135-145, K=3.5-5), C-reactive protein (CRP) (normal range: lower than 3), erythrocyte sedimentation rate (ESR) (normal range: up to 50yrs <15, 50-85yrs <20, >85yrs <30), liver enzymes (normal range: AST=10-40, ALT=7-56, ALP=20-140) and renal function tests (normal range: Creatinine=0.5-1.2, Urea=5-20), lactate dehydrogenase (LDH) (normal range: 140-280), albumin (normal range: 3.4-5.4), and creatine phosphokinase (CPK) (normal range: in male=39-308, in female=26-192) were also performed and recorded. All medical data were kept as a computerized database. Moreover, treatment was started for all the patients according to the existing treatment protocols and daily visits were organized.
Statistical analysis
Distribution of continuous variables were presented by mean (SD) for whole and by median (IQR) for two groups of patients based on their vital status. Categorical variables were presented using frequency (%). We applied negative binomial regression to test the association between clinical variables and length of hospital stay. Incidence Rate Ratio (IRR) and 95%CI was calculated from each model. We used penalized firth logistic regression analysis to test the association of mortality and clinical variables. Odds Ratio (OR) and 95%CI was calculated from each model. IRR and OR were calculated for each unit of continuous variables. All analyses were performed in Stata 16.1 (StataCorp, College Station, TX).
Results
Demographic and clinical characteristics
Fifty-three patients with confirmed COVID-19 infection, admitted to the pulmonary ward between 1 March and 10 April 2020, were enrolled in this study.
The demographic and clinical characteristics are shown in Table 1. A history of recent travel to China, South Korea and Italy, as well as having contact with people from aforementioned countries was not documented in any patients. Five (9.4%) patients had a history of traveling to a high-risk area inside the country. The mean (SD) age of patients was 58.4±13.0 (ranged 30 to 90 years), and 45.3% were males. The median length of stay in hospital was 7 (IQR: 6, 10) days. Fatigue and loss of appetite (60.4%), dyspnea (56.6%), myalgia-arthralgia (52.8%), and dry cough (47.2%) were the most common symptoms of individuals. However, hemoptysis (20.8%), and productive cough (13.2%) were rare presentations. Among patients, Kaletra (Lopinavir / Ritonavir) was prescribed for 20 (43.5%), hydroxychloroquine for 34 (73.9%), interferon for 6 (13%), and methylprednisolone for 16 (35.6%). Furthermore, the most common underlying disease was hypertension (39.6%) followed by diabetes, ischemic heart disease, pulmonary disease and transplantation that were found in 24.5%, 15.1%, 11.3% and 7.5%, respectively. With regard to vital signs, 7.5% of the cases had a pulse rate higher than 100 per minute and pulse rate of lower than 60 per minute was observed in 1.8% of patients. Moreover, only 22.6% of the patients had normal oxygen saturation (higher than 95 percentage). Among patients, only three (5.7%) had a history of influenza vaccination.
Table 1.
Baseline characteristics of the patients and the relationship with length of hospital stay
PR: Pulse rate, AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, ALKP: Alkaline Phosphatase, PLT: Platelet, WBC: White blood cell, NLR: Neutrophil/Lymphocyte Ratio, LDH: Lactate Dehydrogenase, Alb: Albumin, ESR: Erythrocyte Sedimentation Rate, CRP:C-Reactive protein, Cr: Creatinine, Hb: Hemoglobin, PTT: Partial Thromboplastin Time, PT: Prothrombin Time, CPK: Creatine Phosphokinase
IRR: incidence rate ratio, this factor shows the effect of each parameters on the length of hospital stay and is calculated for each unit increase in quantitative variable.
| Factor | Mean ± SD |
IRR (95%CI) |
P-value | Factor | Number(%) |
IRR (95%CI) |
P-value |
| Age | 58.4±13.0 |
0.99 (0.98-1.00) |
0.035 | Gender (male) | 24 (45.3) | 1.18 (0.95-1.45) |
0.127 |
| PR | 87.30±17.14 | 1.00 (0.99-1.01) |
0.304 | Diarrhea (no) | 32 (61.5) |
0.78 (0.64-0.96) |
0.019 |
| O2Sat | 88.4±13.9 | 1.01 (0.99-1.01) |
0.181 | Fatigue(no) | 20 (38.5) | 0.98 (0.79-1.21 |
0.830 |
| AST(U/L) | 46.6±53.5 | 1.00 (0.99-1.00) |
0.444 |
Hemoptysis (no) |
45 (86.5) |
0.73 (0.56-0.96) |
0.024 |
| ALT(U/L) | 49.0±55.0 | 1.00 0.99-1.00) |
0.128 | LOA(no) | 20 (38.5) | 1.05 (0.85-1.29) |
0.663 |
| ALKP (U/L) |
192.5±137.5 | 1.00 (0.99-1.00) |
0.133 | AP(no) | 49 (80.8( | 1.01 (0.78-1.31) |
0.940 |
| PLT | 307451.0±144599.5 | 1.00 (0.99-1.00) |
0.132 | CP(no) | 40 (76.9) | 0.98 (0.77-1.25) |
0.890 |
| WBC | 6801.9±2394.5 | 0.99 (0.99-1.00) |
0.306 | Fever(no) | 38 (73.1) | 1.20 (0.94-1.53) |
0.149 |
| NLR | 3.96±2.73 | 0.97 (0.94-1.01) |
0.214 | Headache(no) | 29 (55.8) |
0.81 (0.66-1.00) |
0.050 |
| LDH (U/L) |
515.2±211.2 | 1.00 (0.99-1.00) |
0.947 | Confusion(no) | 34 (65.4) | 0.93 (0.75-1.15) |
0.499 |
| Alb (g/dL) |
3.45±0.37 | 0.82 (0.61-1.10) |
0.185 | Dry Cough(no) | 27 (51.9) | 0.82 (0.67-1.00) |
0.056 |
| ESR (mm/h) | 50.1±17.9 | 1.00 (0.99-1.01) |
0.663 |
Productive Cough (no) |
41 (78.8) | 0.99 (0.78-1.28) |
0.972 |
| CRP (mg/L) | 41.7±17.8 | 1.00 (0.99-1.01) |
0.609 | Dyspnea(no) | 22 (42.3) | 0.86 (0.70-1.06) |
0.156 |
| Cr (mg/dL) | 1.95±2.15 | 1.02 (0.97-1.06) |
0.458 |
Myalgia/Arthralgia (no) |
24 (46.2) | 1.02 (0.83-1.26) |
0.815 |
| K (mEq/L) | 4.41±0.64 | 0.95 (0.80-1.12) |
0.523 | Sore throat(no) | 42 (80.8) | 0.86 (0.66-1.11) |
0.239 |
| Na (mEq/L) | 139.0±14.7 | 1.00 (0.99-1.01) |
0.698 | HTN(no) | 31 (59.6) | 1.11 (0.90-1.37) |
0.328 |
| Hb(g/dL) | 12.78±1.66 | 0.99 (0.93-1.06) |
0.888 | Nausea(no) | 40 (76.9) | 1.25 (0.97-1.62) |
0.087 |
| HCO3 | 25.1±5.8 | 1.02 (0.99-1.04) |
0.208 | DM(no) | 39 (75.0) | 1.01 (0.80-1.28) |
0.901 |
| PTT (sec) | 21.3±2.9 | 1.01 (0.97-1.05) |
0.662 | Ischemic heart disease(no) | 44 (84.6) | 0.89 (0.68-1.17) |
0.409 |
| PT (sec) | 11.1±0.9 | 1.09 (0.96-1.23) |
0.163 |
Transplantation (no) |
48 (92.3) | 0.88 (0.62-1.27) |
0.512 |
| CPK (U/L) | 152.7±167.8 | 0.99 (0.99-1.00) |
0.813 | Pulmonary disease(no) | 46 (88.5) | 0.95 (0.69-1.29 |
0.738 |
| Urea (mg/dL) | 47.2±43.2 | 0.99 (0.99-1.00) |
0.347 |
Laboratory findings
On admission 5.6%, 15% and 11.3% of patients had lymphocytosis, thrombocytosis and thrombocytopenia, respectively. Elevated levels of CRP (96.2%), ESR (66%), and lactate dehydrogenase (83%) were observed in most individuals. However, elevated level of CPK (11.3%) was rare and decreased level of CPK was found only in 1.8% of patients.
Liver function tests demonstrated high levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) in 35.8%, 18.8% and 47.1% of the patients, respectively. Moreover, according to the renal function tests, increased level of urea (86.7%) was common, but elevated level of creatinine (41.5%), hypernatremia (9.4%), hyponatremia (1.8%), hyperkalemia (7.5%) and hypokalemia (11.3%) were rare. Coagulation tests showed that increased partial thromboplastin time (PTT) (69.8%) was more common than prothrombin time (PT) (11.3%)
Clinical Outcomes
LOHS was significantly associated with clinical symptoms. The absence of some clinical presentations, including hemoptysis (IRR= 0.73, P-value= 0.02), diarrhea (IRR= 0.78, P-value= 0.01), headache (IRR= 0.81, P-value= 0.05) and dry cough (IRR= 0.82, P-value= 0.05) reduced the LOHS. Generally, we did not find any significant relationship between the absence of underlying diseases and LOHS. Also, laboratory tests did not correlate with LOHS.
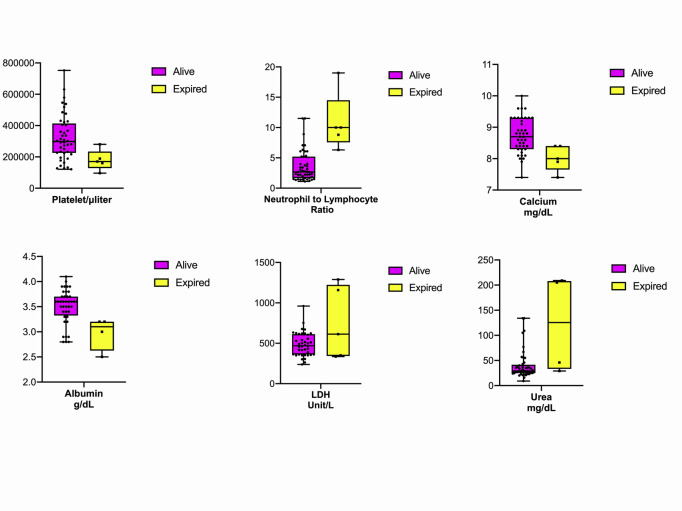
From the included patients 5 of them were deceased (mortality risk= 9.4%). Older age was associated with higher mortality risk (Odds ratio=1.148, 95%CI=1.032-1.276). Decreased levels of oxygen saturation (OR=0.848, 95%CI=0.733-0.982), platelet count (OR=0.999, 95%CI=0.999-1.000), calcium (OR=0.087, 95%CI=0.010-0.788), albumin (OR=0.036, 95%CI=0.002-0.655), as well as with an increased level of neutrophil/lymphocyte ratio (NLR) (OR=1.468, 95%CI=1.086-1.985), LDH (OR=1.004, 95%CI=1.000-1.007), and urea (OR=1.023, 95%CI=1.006-1.039) were associated with higher mortality risk. Figure 1 illustrates the distribution of laboratory tests associated with higher mortality rates among survived and expired patients. (Table 2).
Figure 1.
Distribution of laboratory tests associated with higher mortality rates among survived and expired patients. Platelet count, albumin, and calcium level were higher among survived patients (P value= 0.014, 0.025, 0.030). Neutrophil-to-lymphocyte ratio (NLR), LDH, and Urea were higher among deceased patients (P value= 0.013, 0.026, 0.008).
Table 2.
Relationship between demographic factor and laboratory tests and mortality rates
SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure, PR: Pulse rate, AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, ALKP: Alkaline Phosphatase, PLT: Platelet, WBC: White blood cell, PMN: Polymorphonuclear Neutrophil, NLR: Neutrophil/Lymphocyte Ratio, TG: Triglyceride, Chol: Cholesterol, LDH: Lactate Dehydrogenase, Alb: Albumin, ESR: Erythrocyte Sedimentation Rate, CRP:C-Reactive protein, Cr: Creatinine, Hb: Hemoglobin, HbA1C: HemoglobinA1C, PTT: Partial Thromboplastin Time, PT: Prothrombin Time, CPK: Creatine Phosphokinase
| Factor | Survived | Expired | P-value | Odds ratio | 95% CI | ||
| Median | IQR | Median | IQR | ||||
| Age | 55.5 | 49.6-63.5 | 74.0 | 68.5-86.0 | 0.011 | 1.148 | 1.032-1.276 |
| SBP (mmHg) | 110.0 | 100.0-110.0 | 102.5 | 100.0-105.0 | 0.428 | 0.954 | 0.851-1.071 |
| DBP (mmHg) | 70 | 60-77 | 65 | 60-85 | 0.656 | 1.023 | 0.926-1.130 |
| PR (per minute) | 87.00 | 77.00-97.00 | 86.00 | 69.75-100.75 | 0.943 | 1.002 | 0.946-1.061 |
| T (centigrade) | 36.9 | 36.6-37.2 | 36.3 | 36.2-38.7 | 0.640 | 1.272 | 0.463-3.491 |
| O2 saturation | 91.5 | 88.0-95.0 | 83.0 | 34.5-89.0 | 0.027 | 0.848 | 0.733-0.982 |
| AST (U/L) | 34.0 | 22.0-53.0 | 45.0 | 28.0-71.0 | 0.415 | 1.004 | 0.994-1.014 |
| ALT (U/L) | 32.0 | 20.0-53.0 | 47.5 | 21.7-56.7 | 0.642 | 1.003 | 0.991-1.014 |
| ALKP (U/L) | 164.0 | 118.0-220.0 | 133.0 | 114.0-133.0 | 0.598 | 1.001 | 0.996-1.007 |
| PLT | 297500 | 222250-409250 | 170000 | 128000-234500 | 0.014 | 0.999 | 0.999-1.000 |
| WBC | 6300 | 5225-8100 | 8300 | 6300-9700 | 0.199 | 1.000 | 0.999-1.000 |
| NLR | 2.6 | 1.6-5.2 | 8.8 | 5.2-8.8 | 0.013 | 1.468 | 1.086-1.985 |
| TG (mg/dL) | 145.5 | 111.2-180.0 | 72.5 | 67.0-72.5 | 0.144 | 0.963 | 0.916-1.013 |
| Chol (mg/dL) | 128.5 | 107.5-152.5 | 87.5 | 67.0-87.5 | 0.156 | 0.970 | 0.931-1.011 |
| Ca (mEq/L) | 8.7 | 8.3-9.3 | 8.0 | 7.6-8.4 | 0.030 | 0.087 | 0.010-0.788 |
| LDH (U/L) | 469.5 | 361.2-612.7 | 613.0 | 343.0-1223.5 | 0.026 | 1.004 | 1.000-1.007 |
| HbA1C | 5.8 | 5.5-6.4 | 7.8 | 6.8-7.8 | 0.103 | 1.858 | 0.881-3.918 |
| Alb (g/dL) | 3.6 | 3.3-3.7 | 3.1 | 2.6-3.2 | 0.025 | 0.036 | 0.002-0.655 |
| ESR (mm/h) | 47.0 | 36.5-66.7 | 57.0 | 45.0-57.0 | 0.643 | 1.017 | 0.946-1.094 |
| CRP (mg/L) | 44.5 | 27.7-52.2 | 55.0 | 44.3-64.0 | 0.166 | 1.055 | 0.978-1.138 |
| Cr (mg/dL) | 1.18 | 1.00-1.38 | 1.36 | 1.04-5.60 | 0.194 | 1.206 | 0.909-1.599 |
| K (mEq/L) | 4.40 | 4.05-4.80 | 4.00 | 3.45-4.90 | 0.361 | 0.474 | 0.096-2.352 |
| Hb (g/dL) | 13.0 | 12.0-14.0 | 12.1 | 10.5-13.1 | 0.184 | 0.722 | 0.447-1.167 |
| PCO2 (mmHg) | 44 | 37-49 | 44 | 24-53 | 0.461 | 0.968 | 0.887-1.056 |
| HCO3 | 26.0 | 21.9-29.0 | 27.0 | 16.4-29.8 | 0.586 | 0.958 | 0.821-1.118 |
| PTT (sec) | 20 | 20-22 | 24 | 20-24 | 0.152 | 1.239 | 0.924-1.661 |
| PT (sec) | 10.95 | 10.40-11.50 | 11.70 | 11.20-11.70 | 0.289 | 1.880 | 0.586-6.035 |
| CPK (U/L) | 79.0 | 59.7-134.0 | 206.5 | 79.2-459.0 | 0.184 | 1.003 | 0.999-1.007 |
| Urea (mg/dL) | 29.0 | 25.0-41.5 | 125.5 | 33.2-208.0 | 0.008 | 1.023 | 1.006-1.039 |
| Na (mEq/L) | 141.0 | 138.7-143.0 | 140.0 | 138.0-142.0 | 0.501 | 0.989 | 0.958-1.021 |
| Mg (mEq/L) | 1.9 | 1.6-2.4 | 2.1 | 1.2-3.1 | 0.647 | 1.366 | 0.359-5.194 |
Discussion
The present study has collected a comprehensive data on the number of risk factors for death and longer hospital stays, as well as demographic, clinical, and laboratory characteristics of whom hospitalized as the result of COVID-19 in Iran. Our study identified that decreased level of oxygen saturation, PLT count, calcium, albumin, and increased neutrophil/lymphocyte ratio (NLR), LDH, urea and old age were correlated with higher mortality rates. Also, LOHS was significantly associated with clinical manifestations such as hemoptysis, diarrhea, headache and dry cough.
In the current study, mortality rate was calculated as 9.4%, almost similar to a previous study[13]. Higher fatality was observed in older age group (≥50 years old) affected by the COVID-19, consistent with those in previous reports[14, 15]. Similar to our result Zhou et al. demonstrated that increased age was associated with more deaths in patients with COVID-19[10]. A possible cause of higher mortality in this age group is that the majority of elderly have chronic underlying diseases with mitigated immune respond toward various pathogens.
Concerning the laboratory findings, the absolute count of platelet was reduced in 11.3% patients, whereas, increased partial thromboplastin time (PTT) was more common than prothrombin time (PT). In contrast with these findings, Mi Xiong et al. showed no significant difference in PLT and PTT values in comparison to normal range in SARS-CoV-2 infection[16]. The prolonged PTT in viral infections can be explained by the induced dysfunction of endothelial cells resulting in thrombin generation and fibrinolysis shutdown and this situation may predispose patients to a disseminated intravascular coagulation [17, 18]. Furthermore, we reported the association between higher mortality rates and decreased platelet count, which is similar to a study by Akca et al, that reported thrombocytopenia as a predictive factor for death. They also showed that the differences of PLT count varied in survivors and non-survivor in critically ill patients who were admitted to intensive care unit (ICU)[19].
Consistent with prior findings[11, 20], increased neutrophil/lymphocyte ratio(NLR) had a relationship with higher mortality rates. Increased NLR may be due to cytokine storm, series of immune responses, and peripheral white blood cell changes in the setting of SARS-CoV2 infection giving rise to a more critical condition.
Additionally, our result revealed a correlation between higher mortality rates and increased level of LDH. Similar to our findings, Han et al. showed that LDH was a powerful predictive factor for lung injury and severe COVID-19 cases[21]. The aforementioned finding might be due to the positive association between LDH and CRP which was observed in severe SARS-CoV-2 cases[22]. Moreover, elevated levels of LDH leads to activation of immune-suppressive cells, such as macrophages and dendritic cells (DCs), as well as inhibition of cytotoxic cells, such as natural killer (NK) cells and cytotoxic T-lymphocytes (CTLs)[23], resulting in a more critical situation.
The current study revealed that hypocalcemia was a critical risk factor for COVID-19 mortality. Previous studies have identified that patients with hypocalcemia were more susceptible to death, during sepsis or other medical conditions[24, 25]. Likewise, an elevated level of serum urea could increase mortality in the present study. Xiang et al. observed that serum urea in severe COVID-19 patients was significantly higher than those in mild conditions[26].
Additionally, 67% of our patients had underlying diseases. Findings from previous studies[27] showed that hypertension and diabetes were the most common underlying diseases among COVID-19 subjects. The possible reason could be the increased expression of angiotensin-converting enzyme 2 (ACE2) in patients with type 1 or type 2 diabetes and hypertension, who are treated with ACE inhibitors and angiotensin II type-I receptor blockers (ARBs). As a result, the increased expression of ACE2 would facilitate the development of COVID-19 infection[28, 29]. Moreover, the most notable factor related to the mortality rate was hypoalbuminemia, according to our results. In a similar way, many researches noticed that lower albumin level could increase the mortality rate[30, 31]. This may be due to the fact that albumin production downregulates the expression of the ACE2 receptors[32], which is the primary receptor for the SARS-CoV-2 virus.
In terms of clinical presentations, dry cough and dyspnea were the most common symptoms, while hemoptysis, and productive cough were rare presentations. This may be due to viral tropism in COVID-19 infection similar to that of SARS-CoV and MERS-CoV[33, 34]. This presentation is observed as the consequence of diffuse alveolar damage with hyaline membrane formation, alveolar wall inflammation, and desquamation of pneumocytes[34].
Turning to the strengths and limitations of this study, to the best of our knowledge, this is the first report to explore the risk factors, laboratory findings and clinical manifestations among COVID-19 patients in the Iranian population. A number of limitations could be mentioned. First is the small sample size, which might affect the observed correlations. Second, the retrospective nature of this study makes it hard to consider definite causality between the risk factors and outcome.
Conclusion
The COVID-19 infection, initially reported in Wuhan, China, has been declared a global health emergency. Presence of diarrhea, hemoptysis, headache and dry cough were potential risk factors for a longer hospital stay in our patients. Decreased levels of O2 saturation, platelet count, calcium, and albumin could accompany higher mortality rates. However, increased neutrophil/lymphocyte ratio (NLR), LDH, urea, and old age were associated with higher mortality rates among patients.
Acknowledgment
None to declare
Conflict of Interest Disclosure
None to declare
References
- 1.Huang C, Huang C, Wang Y, Li X, et al. Clinical features of patients infected with. 2019:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins C. Coronavirus COVID-19 Global Cases. 2020 [Google Scholar]
- 3.Musa T.H, et al. Global outbreak of 2019-nCoV, a new challenge? The Journal of Infection in Developing Countries. 2020;14(03):244–245. doi: 10.3855/jidc.12530. [DOI] [PubMed] [Google Scholar]
- 4.Lu R, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richman D.D, Whitley R.J, Hayden F.G. Clinical virology. John Wiley & Sons: 2016. [Google Scholar]
- 6.Singhal T. A review of coronavirus disease-2019 (COVID-19) The Indian Journal of Pediatrics. 2020:1–6. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox S.E, et al. Pulmonary and Cardiac Pathology in Covid-19: The First Autopsy Series from New Orleans. MedRxiv. 2020 doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onder G., G. Rezza, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. Jama. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, et al. Clinical progression of patients with COVID-19 in Shanghai, China. Journal of Infection. 2020 doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, et al. Perioperative care provider’s considerations in managing patients with the COVID-19 infections. Transl Perioper Pain Med. 2020;7:216–223. [Google Scholar]
- 12.Li Q, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England Journal of Medicine. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVID C, Team R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohrabi C, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) International Journal of Surgery. 2020 doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong M, Liang X, Wei Y.D. Changes in Blood Coagulation in Patients with Severe Coronavirus Disease 2019 (COVID-19): a Meta-Analysis. Br J Haematol. 2020 doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt F.C.F, et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Annals of intensive care. 2019;9(1):19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han H, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clinical Chemistry and Laboratory Medicine (CCLM) 2020:1. doi: 10.1515/cclm-2020-0188. ahead-of-print. [DOI] [PubMed] [Google Scholar]
- 19.Akca S, et al. Time course of platelet counts in critically ill patients. Critical care medicine. 2002;30(4):753–756. doi: 10.1097/00003246-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Tan L, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal transduction and targeted therapy. 2020;5(1):1–3. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Y, et al. Lactate dehydrogenase, a Risk Factor of Severe COVID-19 Patients. medRxiv. 2020 doi: 10.18632/aging.103372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding J, Karp J.E, Emadi A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomarkers. 2017;19(4):353–363. doi: 10.3233/CBM-160336. [DOI] [PubMed] [Google Scholar]
- 24.Zaloga G.P, Chernow B. The multifactorial basis for hypocalcemia during sepsis: Studies of the parathyroid hormone-vitamin D axis. Annals of internal medicine. 1987;107(1):36–41. doi: 10.7326/0003-4819-107-1-36. [DOI] [PubMed] [Google Scholar]
- 25.Chernow B, et al. Hypocalcemia in critically ill patients. Critical care medicine. 1982;10(12):848–851. doi: 10.1097/00003246-198212000-00008. [DOI] [PubMed] [Google Scholar]
- 26.XIANG J, et al. Potential biochemical markers to identify severe cases among COVID-19 patients. medRxiv. 2020 2020.03.19.20034447. [Google Scholar]
- 27.Fang L.G, Karakiulakis , Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet. Respiratory Medicine. 2020 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan Y, et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. Journal of virology. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X.C, Zhang J, Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacological research. 2017;125:21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinhardt G.F, et al. Incidence and mortality of hypoalbuminemic patients in hospitalized veterans. Journal of Parenteral and Enteral Nutrition. 1980;4(4):357–359. doi: 10.1177/014860718000400404. [DOI] [PubMed] [Google Scholar]
- 31.Watkins J. Preventing a covid-19 pandemic. BMJ. 2020;368:m810. doi: 10.1136/bmj.m810. [DOI] [PubMed] [Google Scholar]
- 32.Liu B.C, et al. Albumin caused the increasing production of angiotensin II due to the dysregulation of ACE/ACE2 expression in HK2 cells. Clin Chim Acta. 2009;403(1-2):23–30. doi: 10.1016/j.cca.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Assiri A, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. New England Journal of Medicine. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guarner J. Three Emerging Coronaviruses in Two Decades: The Story of SARS, MERS, and Now COVID-19. American Journal of Clinical Pathology. 2020;153(4):420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]