Abstract
Obstructive sleep apnea (OSA) is a broadly diffused curable chronic low-grade inflammatory disease sharing impressive clinical and pathogenetic features with Covid-19. Moreover, a potential role of OSA as a detrimental factor for Covid-19 severity has been hypothesized. Continuous positive airway pressure (CPAP) is the mainstay treatment for moderate-severe OSA, but the beneficial effects of ventilation strongly depend on medical expertise and on the patient’s adherence and compliance. Although several papers have analyzed the overlaps and outcomes of OSA and Covid-19, limited attention has been dedicated to ventilatory adherence and management of OSA cohorts exposed to Covid-19. We briefly review the literature data, pointing out the main risks and benefits of CPAP for OSA patients in the pandemic setting. (www.actabiomedica.it)
Keywords: obstructive sleep apnea syndrome, Covid-19, inflammation, non-invasive ventilation, CPAP
Introduction
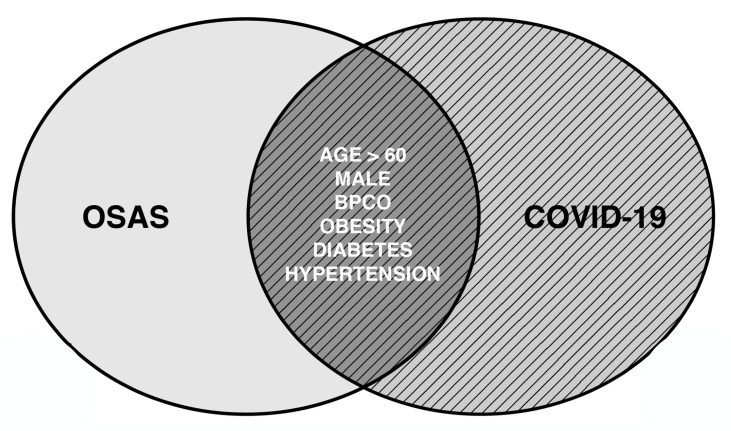
The entire world is fighting against a novel coronavirus called Sars-Cov2 responsible for bilateral infiltrative pneumonia, which may lead to severe respiratory insufficiency1. The WHO named the disease Covid-19. Demonstrated risk factors for Covid-19 include obesity, diabetes, hypertension, older age and male sex2. Attention has been focused also on the potential role of obstructive sleep apnea (OSA) as a detrimental factor for disease severity. To date, Pubmed search strategy based on keywords ((sleep apnea) AND (covid)) OR ((OSA) AND (covid)), offers 73 articles, mainly composed of letters to editor, recommendations and brief reviews, with only a limited amount of original articles of variable sample sizes, in many cases sharing information collected through phone calls or indirect patient evaluation. Most of the cited papers analyze the clinical commonalities between OSA and Covid-19: indeed an impressive overlap between the canonical characteristics of Sars-Cov2 and OSA is often recognizable (Fig. 1). The prototypical subject affected by severe Covid-19 is an obese middle-aged/elderly male suffering from a metabolic syndrome: similar features are major risk factors for OSA development.
Figure 1.
Overlapping features of obstructive sleep apnea and Covid-19
Pathophysiology of increased risk in OSA patients
Variable underlying mechanisms are responsible for higher rates of disease complication in patients affected by OSA, a risk factor for community-acquired pneumonia and viral infections. OSA is also linked to pneumonia severity, it can predispose to increased aspiration and may compromise the efficacy of the defensive cough reflex3. During influenza epidemics, OSA can become a risk factor for ICU admission3 and it is associated with higher concentration of PAI-1, a component of the coagulation system correlated with increased risk for acute vascular events4. Untreated OSA patients present dysregulation of the renin-angiotensin system with over-expression of angiotensin-converting enzyme 2, a well-known entry receptor for Sars-Cov2, likely contributing to higher vulnerability for Covid-19. Indeed the rapid clinical deterioration in Covid-19 has been linked to life-threatening coagulation dysfunction together with the so-called cytokine storm and the Covid-19 clinical story is often accompanied by thrombosis, stroke and arrhythmia, cardiac injury, fulminant myocarditis, heart failure, pulmonary embolism, and disseminated intravascular coagulation5.
OSA has been described as a pre-existent pathology in up to 28% (6/21 patients) of Covid-19 patients in an ICU cohort6. Acting as an independent risk factor for disease severity, OSA increases the risk for hospitalization7 and is related to 7-days mortality8.
Benefits from CPAP utilization
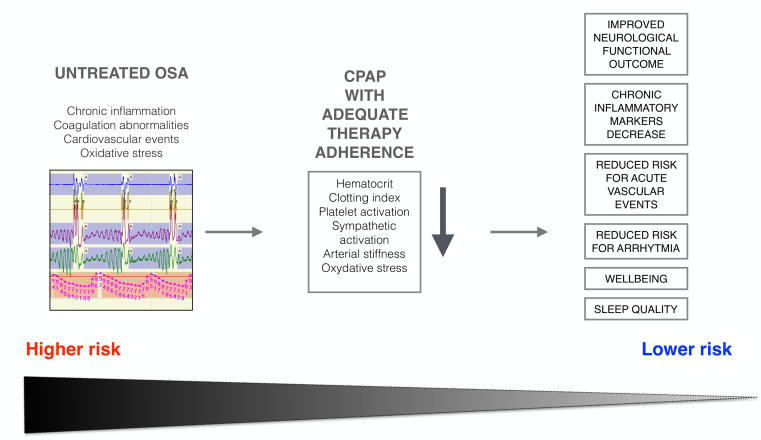
Despite the relevant papers on the comorbidity between sleep apnea and Covid-19 (in many cases based on the ICDS code of health care registers), limited attention has been dedicated to the complex issues regarding treatment strategies. Compared to other unchangeable risk factors, OSA can be easily identified and managed with a variety of approaches. In particular, non-invasive ventilation, e.g., continuous positive airway pressure (CPAP) is the mainstay for treatment of moderate-to-severe OSA patients. CPAP promotes variable cardiovascular protective effects9, reduces hypercoagulability decreasing platelet activation, clot strength, hematocrit and lowering blood viscosity. It also increases functional residual capacity thereby improving gas exchange10. Furthermore, benefits on arterial stiffness and reduction of serum inflammatory markers including IL-2, IL-4, IL-6, MCP-1, PDGFβ, and VEGFα can be appreciated in severe OSA patients using non-invasive ventilation for at least 4-hours/night11,12. Reduced mRNA expression of markers related to tissue hypoxia and macrophage infiltration, such as ATF4, CHOP, ERO-1, CD68, CD36, IL-6, PDGFβ have also been described11. Accordingly, CPAP reduces incidence of acute vascular events and improves post-stroke functional outcome among OSA patients adherent to treatment13 (Fig. 2). Beneficial effects of CPAP in OSA patients are recognizable since the very beginning of therapy, but strongly depend on patient’s adherence and compliance to therapy. Solid data confirmed the importance of regular follow-up to guarantee treatment efficacy: a minimum of 4-h/night is mandatory for at least 70% of nights, with best results when CPAP is used for at least 6-hours/night14.
Figure 2.
Impact of well-conducted non-invasive ventilation with CPAP on OSA risk factors.
Safe use of CPAP devices
Current available recommendations from international societies15,16 underline risks related to the utilization of PAP devices in terms of increased virus transmission through aerosol and droplets generation with possible environmental contamination. In the current pandemic background, clinical trends postpone OSA diagnosis and PAP titration rather than starting therapy immediately and keeping adequate follow-up plans. However, relevant scientific contributions demonstrate that filters protect from viral diffusion: using a well-fitted oronasal mask results in a negligible dispersion of aerosol at pressures from 5 to even up to 20 cmH20. Set-up anti-viral filters prevent bacteria and viruses from entering the CPAP tubes and masks, protecting the patients’ airway without influencing air pressure from CPAP machine. CPAP masks may be divided into vented and non-vented masks: non-vented masks with an exhalation valve are to prefer to vented masks as they avoid breathing autocycle, minimizing droplets dispersions and allowing proper release of carbon dioxide. For OSA patients with co-existent CODP or restrictive lung diseases bilevel positive airway pressure (BiPAP) is a potential alternative therapy: in these cases, a double lumen tube with viral/bacterial filter and non-vented mask is advisable. These suggestions can be applied both in the hospital setting as well as in the domestic environment (Tab. 1).
Table 1.
Everyday hygiene advisories for CPAP users
| Apply a viral/bacterial filter on the machine |
| Change machine viral/bacterial filter at least after 24 h of utilization |
| Clean masks and tubes with hand-hot soap water |
| Wash your hands with soap and water before and after handling the CPAP device |
| Ensure well-fitting of oro-nasal mask to minimize air leaks |
| Prefer the utilization of a non-vented mask |
Importance of adherence to therapy
To the best of our knowledge, the published material exploring the epidemiological link between OSA and Covid-19 neglects the role of chronic non-invasive ventilation nor tries to stratify results according to patients’ adherence to treatment. In a pathology with manifold dynamics including patients’ compliance, available information regarding efficacy and adherence to treatment is mandatory to understand the relationship between OSA and Covid-19. Accordingly, exploring whether well-performed therapy lowers the risk for complications related to Covid-19 in OSA patients becomes a topical issue. Unfortunately, adherence to therapy in OSA samples has slightly decreased in the last months: in a telephone survey performed in New York City around 11% of OSA patients declared that they had ceased to use their PAP device, reflecting the difficulties for sleep clinicians to maintain adequate connection to outpatients17. Updated data from other countries, exploring patients’ compliance during the pandemia, are desirable. It is known that CPAP discontinuation may lead to relapses including lethargy, headache, confusion and dyspnoea18. Therefore, control of PAP devices can allow timely identification of changes in breathing patterns, which may be useful for an early identification of latent viral infections, integrating available screening procedures19.
Conclusion and perspectives
Given the multiple and immediate beneficial role of CPAP in reducing risks for infectious complications, chronic inflammation and thrombophilia in OSA patients and considering the relative safety of PAP when adequate precautions are satisfied, we suggest that involved specialists should work on diagnosis of high-risk cases using safe procedures (gloves, full face shields and others personal protective equipment), starting therapy in newly diagnosed patients with appropriate PAP devices and masks (well-fitting non-vented masks and antiviral filters should be preferred), encouraging regular follow-up (warranting routinely machine hygiene) and promoting adherence with either telemedicine or direct contacts if necessary.
Even if each country will deal with changing situations, constantly adjusting to emergencies and unpredictable necessities, there is urgent need for harmonized protocols to guide OSA management during this era of pandemia.20 To shed light on the OSA and Covid-19 interaction, wide-sample studies analyzing the significance of well-conducted chronic ventilation therapy shall be carefully explored, focusing on CPAP adherence and efficacy, since the importance of patients compliance and the existence of a dose-response relationship between CPAP and health state benefits is a pivotal issue9. Should these premises be confirmed, enhanced screening programs to guarantee prompt OSA management and protocols of behavioral intervention and/or patient coaching to encourage stronger adherence to therapy may reduce the need for intensive care support during the present and future infective outbreaks, upgrading health care standards.
Acknoledgements
none
Conflict of interest
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beumer MC, Koch RM, van Beuningen D, et al. Influenza virus and factors that are associated with ICU admission, pulmonary co-infections and ICU mortality. J Crit Care. 2019;50:59–65. doi: 10.1016/j.jcrc.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes. 2013;37:333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 5.Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020 Aug 1;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arentz M, Yim E, Klaff L, et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. doi:10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maas M.B, Kim M, Malkani R.G, et al. Obstructive Sleep Apnea and Risk of COVID-19 Infection, Hospitalization and Respiratory Failure. Sleep Breath. 2020 doi: 10.1007/s11325-020-02203-0. https://doi.org/10.1007/s11325-020-02203-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. https://doi.org/10.1007/s00125-020-05180-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross MD, Mills NL, Al-Abri M, et al. Continuous positive airway pressure improves vascular function in obstructive sleep apnoea/ hypopnoea syndrome: a randomized controlled trial. Thorax. 2008;63:578–83. doi: 10.1136/thx.2007.081877. [DOI] [PubMed] [Google Scholar]
- 10.Lindner KH, Lotz P, Ahnefeld FW. Continuous positive airway pressure effect on functional residual capacity, vital capacity and its subdivisions. Chest. 1987;92:66–70. doi: 10.1378/chest.92.1.66. [DOI] [PubMed] [Google Scholar]
- 11.Ning Y, Zhang TS, Wen WW, et al. Effects of continuous positive airway pressure on cardiovascular biomarkers in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. Sleep Breath. 2019 Mar;23:77–86. doi: 10.1007/s11325-018-1662-2. [DOI] [PubMed] [Google Scholar]
- 12.Perrini S, Cignarelli A, Quaranta VN, et al. Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea. JCI Insight. 2017 Sep 7;2(17):e94379. doi: 10.1172/jci.insight.94379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau HL, Rundek T, Ramos AR. Sleep and Stroke: New Updates on Epidemiology, Pathophysiology, Assessment, and Treatment. Curr Sleep Med Rep. 2019 Jun;5:71–82. doi: 10.1007/s40675-019-00142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer AM, Goonerathne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med. Rev. 2011;15:343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond M. Sleep labs, lung function tests and COVID-19 pandemic - Only emergencies allowed. Pulmonology. 2020 Jul-Aug;26:244–245. doi: 10.1016/j.pulmoe.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker J, Oyefeso O, Koeckerling D, Mudalige NL, Pan D. COVID-19: community CPAP and NIV should be stopped unless medically necessary to support life. Thorax. 2020 May;75:367. doi: 10.1136/thoraxjnl-2020-214890. [DOI] [PubMed] [Google Scholar]
- 17.Thorpy M, Figuera-Losada M, Ahmed I, et al. Management of sleep apnea in New York City during the COVID-19 pandemic. Sleep Med. 2020;74:86–90. doi: 10.1016/j.sleep.2020.07.013. doi:10.1016/j.sleep.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker JG, Sovani M. Case for continuing community NIV and CPAP during the COVID-19 epidemic. Thorax. 2020 May;75(5):368. doi: 10.1136/thoraxjnl-2020-214913. doi: 10.1136/thoraxjnl-2020-214913. Epub 2020 Apr 9. PMID: 32273336. [DOI] [PubMed] [Google Scholar]
- 19.Nakano H, Kadowaki M, Furukawa T, Yoshida M. Rise in nocturnal respiratory rate during CPAP may be an early sign of COVID-19 in patients with obstructive sleep apnea. J Clin Sleep Med. 2020 Jul 28 doi: 10.5664/jcsm.8714. jc-20-00338. Epub ahead of print. PMID: 32720641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voulgaris A, Ferini-Strambi L, Steiropoulos P. Sleep medicine and COVID-19. Has a new era begun? Sleep Med. 2020 Sep;73:170–176. doi: 10.1016/j.sleep.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]