FIGURE 1.
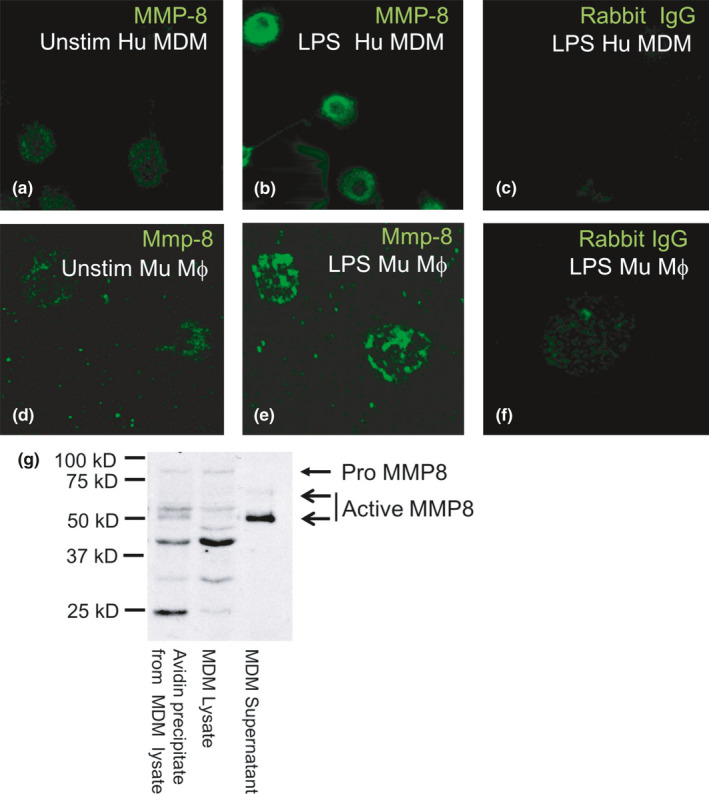
MMP‐8 is localized on the surface of mononuclear phagocytes, as assessed by confocal microscopy and biotinylation of surface proteins. Confocal micrographs show human (Hu) monocyte‐derived macrophages (MDM; a–c) and murine macrophages (Mu Mϕ; d–f) grown on coverslips that were either not stimulated (Unstim) or incubated for 24 h with bacterial lipopolysaccharide (LPS) and then fixed (but not permeabilized) and immunostained with a green fluorophore for surface MMP‐8. There was low‐level MMP‐8 staining on the surface of unstimulated human MDM and murine macrophages (a and d) and increased staining for MMP‐8 on LPS‐activated cells (b and e). Cells incubated with the isotype‐matched control antibody show minimal non‐specific staining (c and f). (g) Proteins on the surface of human MDM or proteins released by the cells were labeled with biotin. Unbound biotin was quenched with glycine, and cells and cell‐free supernatant fluids were separated by centrifugation. Cells were lyzed, and surface biotinylated proteins were captured using immobilized avidin, separated by SDS‐PAGE, and then immunoblotted with a murine monoclonal anti‐MMP‐8 antibody, which recognizes pro‐, active, and further processed MMP‐8. Biotinylated surface proteins of LPS‐stimulated macrophages avidin‐precipitated from cell lysates (lane 1, 50 µL) were compared to total cell lysate (removed before the avidin precipitation step; lane 2, 50 µL) and cell supernatant that was not subjected to avidin precipitation (lane 3, 50 µL) by western blotting with a monoclonal mouse anti‐MMP‐8 antibody. Bands corresponding to pro and active MMP‐8 are indicated by the arrows. Note that surface biotinylation of MDM demonstrates that most of the MMP‐8 forms that are present in macrophage lysates are also detected on the surface of the cells. The results shown are representative of 3 separate experiments
