Fig. 1 |. Inducers of endoplasmic reticulum stress in the tumour microenvironment.
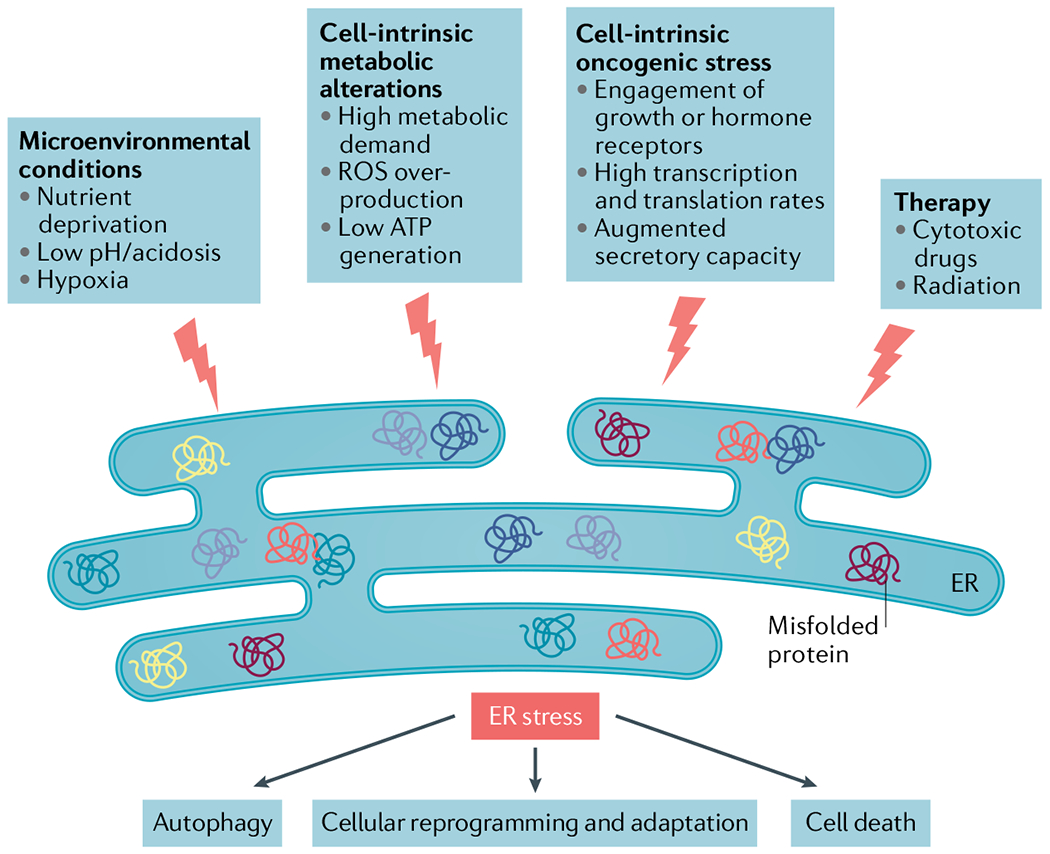
The uncontrolled proliferative capacity of malignant cells in growing tumours engenders hostile microenvironments characterized by high metabolic demand, hypoxia, nutrient limitations and acidosis, which in turn provoke disruption of calcium and lipid homeostasis in multiple cell types inhabiting this milieu. Collectively, these harsh conditions alter the protein-folding capacity of the endoplasmic reticulum (ER) in both cancer cells and infiltrating immune cells, thereby promoting accumulation of misfolded or unfolded proteins within this organelle and, consequently, ER stress. Oncogenic events in cancer cells further contribute to this state by elevating their global transcription and translation rates. The unfolded protein response (UPR) is subsequently activated as an attempt to restore ER homeostasis and promote adaptation to diverse insults in the tumour. Certain therapeutic modalities can also trigger ER stress in the cancer cell to alter their normal behaviour in the tumour microenvironment (TME). Depending on the magnitude of ER stress, the cell type and the specific pathological context, ER stress responses can have multiple effects ranging from cellular reprogramming and adaptation to autophagy and apoptosis. Owing to the additive effects of various ER stressors concurrently enriched in the TME during cancer initiation, progression and therapy, robust and persistent UPR activation is mostly evidenced in cancer cells and tumour-infiltrating immune cells in vivo, which has been challenging to recapitulate under in vitro conditions. ROS, reactive oxygen species.
