Fig. 3 |. Integration of oncogenic programmes and endoplasmic reticulum stress responses in the cancer cell.
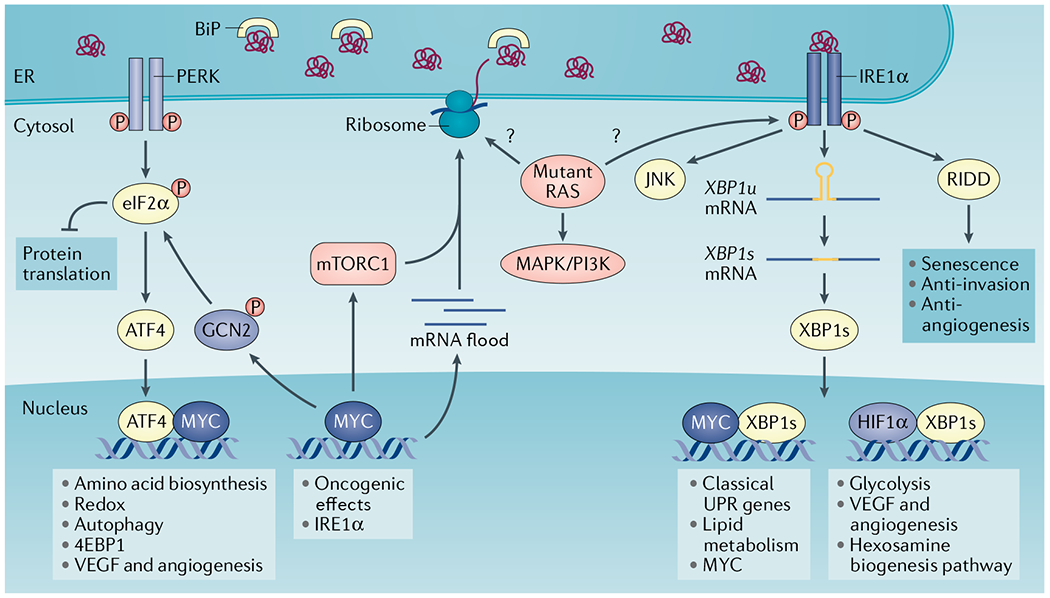
Oncogenic MYC activates the unfolded protein response (UPR) through multiple mechanisms. MYC-induced upregulation of global transcription (mRNA flood) and translation increases ribosome biogenesis and protein load in the endoplasmic reticulum (ER), thus activating all branches of the UPR. MYC further binds to promoter and enhancer regions in the gene encoding inositol-requiring protein 1α (IRE1α), positively regulating its transcription and augmenting IRE1α protein levels. MYC can also form a heterodimer with X-box binding protein 1s (XBP1s) in the nucleus to regulate classical UPR genes and lipid metabolism genes. Of note, XBP1s has been shown to promote MYC transcription in prostate cancer cells and natural killer cells. MYC engages PRKR-like ER kinase (PERK) and general control non-derepressible 2 (GCN2) kinase to induce eukaryotic translation initiation factor 2α (eIF2α) phosphorylation and the integrated stress response. MYC can also interact with activating transcription factor 4 (ATF4) to regulate amino acid transporters and biosynthesis, antioxidant pathways and autophagy. The MYC–ATF4 complex regulates eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) to reduce translation and proteotoxic stress. mTOR complex 1 (mTORC1) activation induces protein synthesis and ER overload that activates the UPR. In turn, the IRE1α–tumour necrosis factor receptor-associated factor 2 (TRAF2)–JUN N-terminal kinase (JNK)–insulin receptor substrate 1 (IRS1) axis has been shown to restrict mTORC1 activity. Mutant RAS is integrated within the UPR in a context-specific manner. Mutant HRAS preferentially induces IRE1α activity in keratinocytes through an unknown mechanism. In primary human melanocytes, HRAS-G12V–PI3K, but not BRAF-V600E, increases ER content and induces activation of all UPR branches. It is unclear whether mutant RAS enhances global protein translation and protein load in the ER, which promotes ER stress in all cancer types. BiP, binding-immunoglobulin protein; HIF1α, hypoxia-inducible factor 1α; P, phosphorylation; RIDD, regulated IRE1-dependent decay of RNA; VEGF, vascular endothelial growth factor.
