Fig. 4 |. Immunomodulatory effects of endoplasmic reticulum stress signals in the tumour microenvironment.
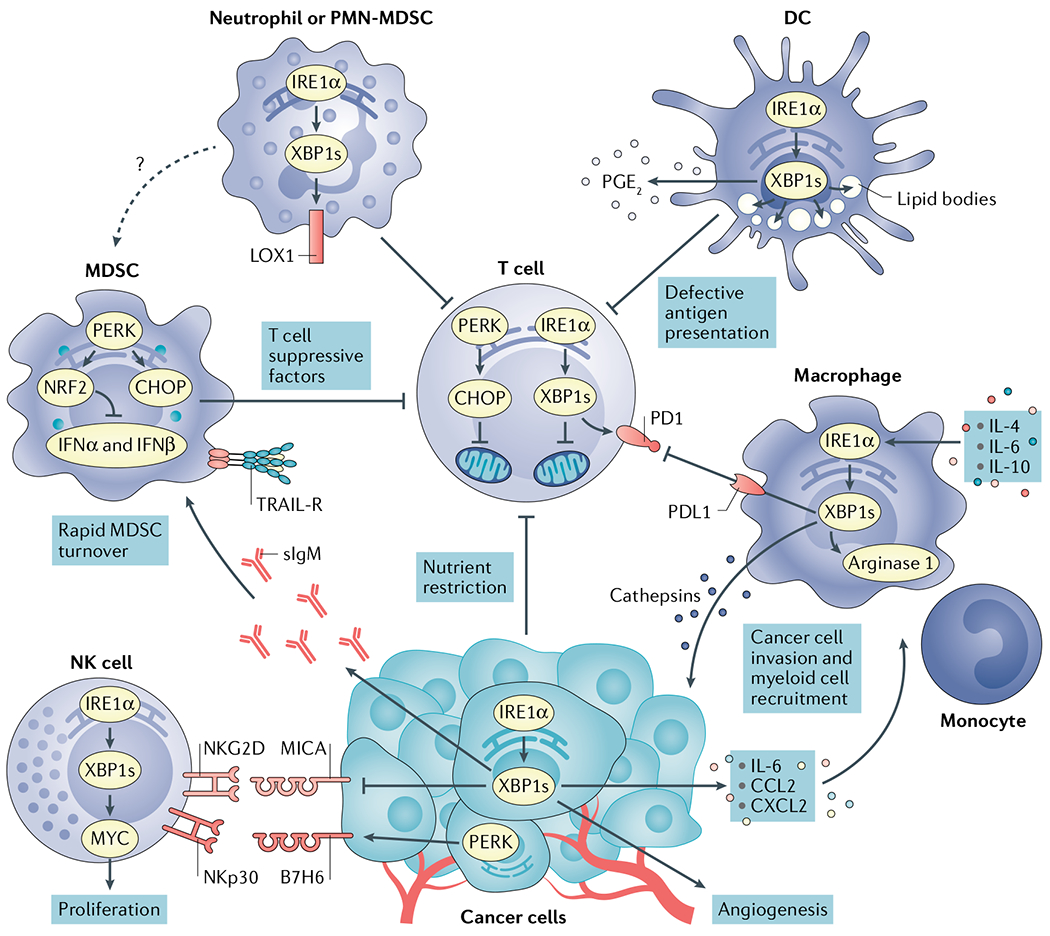
Cancer cells undergoing activation of inositol-requiring protein 1α (IRE1α) or PRKR-like ER kinase (PERK) modulate tumour recognition by natural killer (NK) cells while secreting mediators that promote angiogenesis and recruitment of myeloid cell types to tumour sites. Both IRE1α and PERK are well established to regulate angiogenesis168. X-box binding protein 1s (XBP1s) and activating transcription factor 4 (ATF4) directly bind to the vascular endothelial growth factor (VEGF) promoter to regulate its expression. Nutrient restriction, reactive oxygen species (ROS) accumulation or the presence of soluble factors that blunt glucose uptake cause endoplasmic reticulum (ER) stress and chronic activation of the IRE1α–XBP1 and PERK–C/EBP homologous protein (CHOP) arms of the unfolded protein response (UPR) in intratumoural T cells, provoking mitochondrial dysfunction and inhibition of their optimal anticancer effector function. High levels of cholesterol in the tumour microenvironment (TME) can also activate IRE1α–XBP1 signalling in intratumoural T cells to induce programmed cell death protein 1 (PD1) expression and limit their protective activity. Myeloid-derived suppressor cells (MDSCs) exploit PERK to control antitumour immunity via CHOP-mediated expression of T cell suppressive factors and by inducing nuclear factor erythroid 2-related factor 2 (NRF2)-driven responses that inhibit production of protective type I interferon. ER stress in MDSCs has also been associated with their elevated expression of tumour necrosis factor-related apoptosis-inducing ligand-receptors (TRAIL-Rs) and rapid turnover in the TME. ER stress-related gene signatures and expression of lectin-type oxidized LDL receptor 1 (LOX1) distinguish normal neutrophils from polymorphonuclear (PMN)-MDSCs in patients with cancer. In addition, ER-stressed neutrophils acquire immunosuppressive attributes and overexpress LOX1 via IRE1α activation. ROS accumulation fuels ER stress and persistent IRE1α–XBP1 activation in tumour-associated dendritic cells (DCs), driving uncontrolled lipid droplet formation that inhibits their capacity to present local antigens to intratumoural T cells. ER-stressed DCs have also been shown to overproduce the immunosuppressive lipid mediator prostaglandin E2 (PGE2) via IRE1α–XBP1 activation, presumably contributing to immune escape in cancer. In macrophages, the IRE1α–XBP1 branch has been shown to promote expression of cathepsins, PD1 ligand 1 (PDL1) and Arginase 1, further promoting cancer cell invasion and immunosuppression in the TME. CCL2, CC-chemokine ligand 2; CXCL2, CXC-chemokine ligand 2; IFNα, interferon-α; IL-6, interleukin-6; MICA, MHC class I polypeptide-related sequence A; NKGD2, natural killer group 2D; sIgM, secretory immunoglobulin M.
