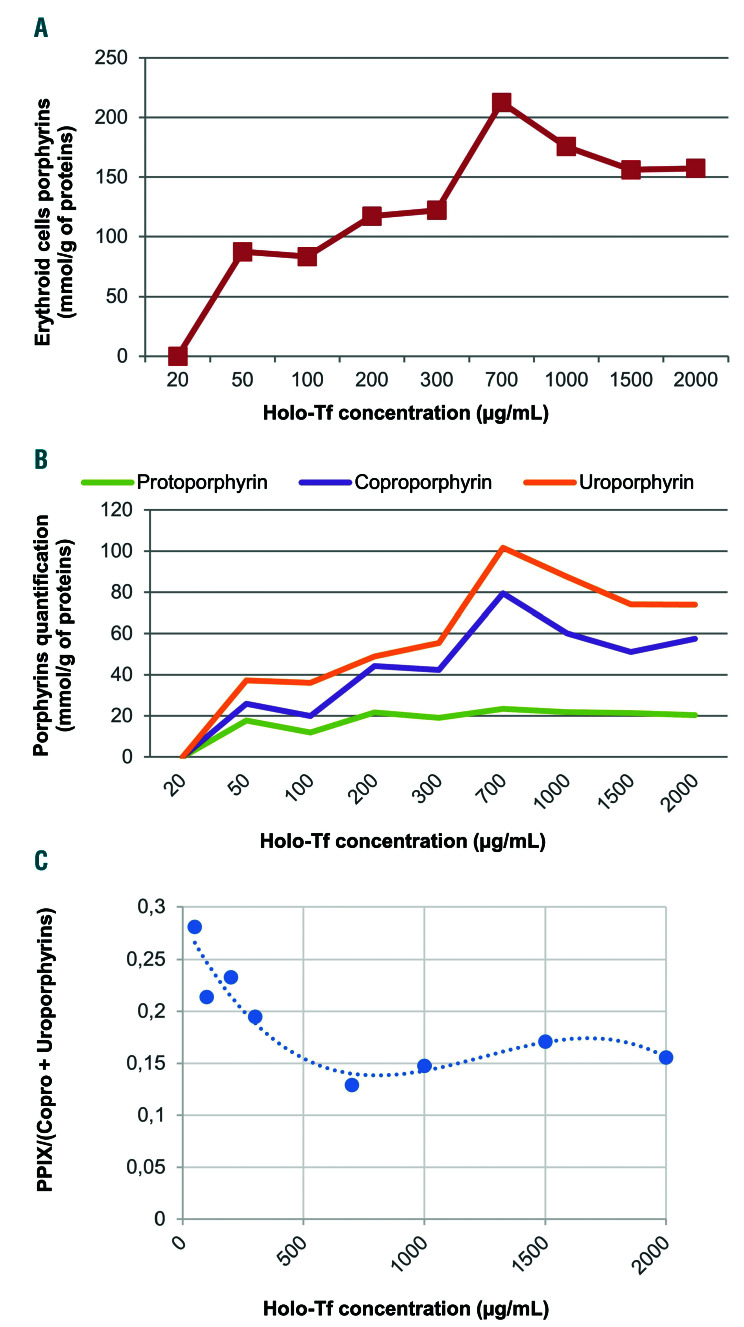
Figure 3.
In vitro erythroid cell porphyrin analysis. Cells were harvested on day 18 for assessment of total erythroid cell porphyrins and porphyrin separation by high performance liquid chromatography with fluorescence detection. Erythrocyte, plasma and urine porphyrins were measured as previously described.15 (A) Porphyrin levels in erythroid cells derived from peripheral CD34+ cells, normalized by protein content after culture in increasing concentrations of holo-transferrin (Holo-Tf). Porphyrins were not detectable in the 20 mg/mL condition, presumably because of the technique’s insufficient sensitivity. (B) Quantification and separation of the different types of porphyrins in erythroid cells by chromatography. (C) The ratio of protoporphyrin IX (PPIX) to coproporphyrin (Copro) level plus uroproporphyrin level ranged from approximately 0.3 in iron-depleted conditions to 0.15 in iron-replete conditions.