Figure 1.
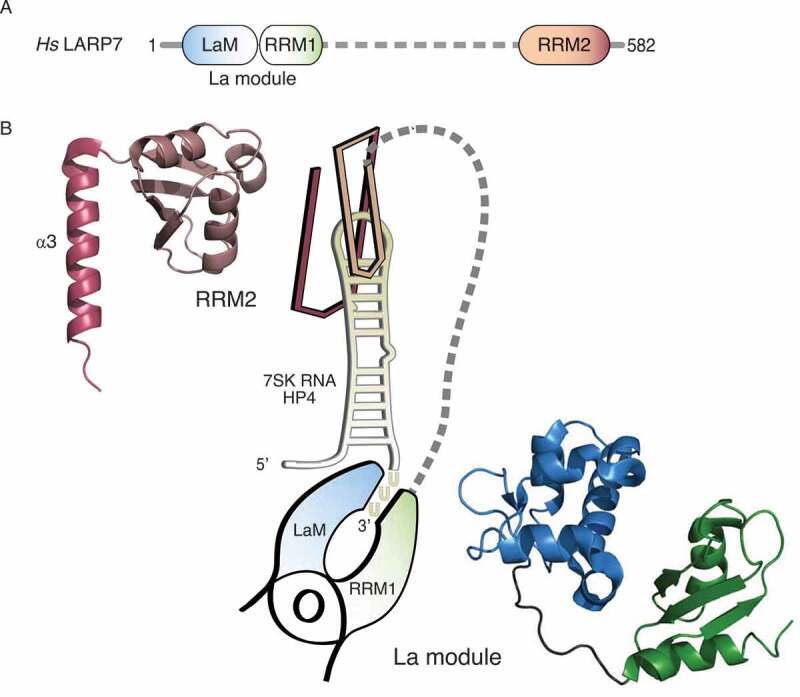
Two RNA-binding platforms within the LARP7 protein mediate contacts to RNA. (A) Schematic overview of the domain organization of human LARP7. The N-terminal La motif (LaM, blue) and the adjacent RNA recognition motif (RRM1, green) form the so-called La module, the common hallmark of LARPs. A flexible linker (dashed line, grey) connects it to the C-terminal RRM2 (salmon). (B) Graphical representation of the binding of LARP7 to terminal hairpin of the 7SK RNA (HP4, yellow) according to the structural studies by Uchikawa et al. (2015) and Eichhorn et al. (2018). The La module is represented as a clamp pinching the single-stranded 3ʹ end of HP4, the RRM2 as a clip inserted on top of the apical loop. The corresponding structures are shown at the sides and were depicted using PyMOL (https://pymol.org/2/) and the data deposited in the Protein Data Bank (PDB): 4WKR for the La module and 6D12 for the RRM2, starting from residue 456. Colours as in (A) with the α3-helix highlighted by darker colouring
