Figure 4.
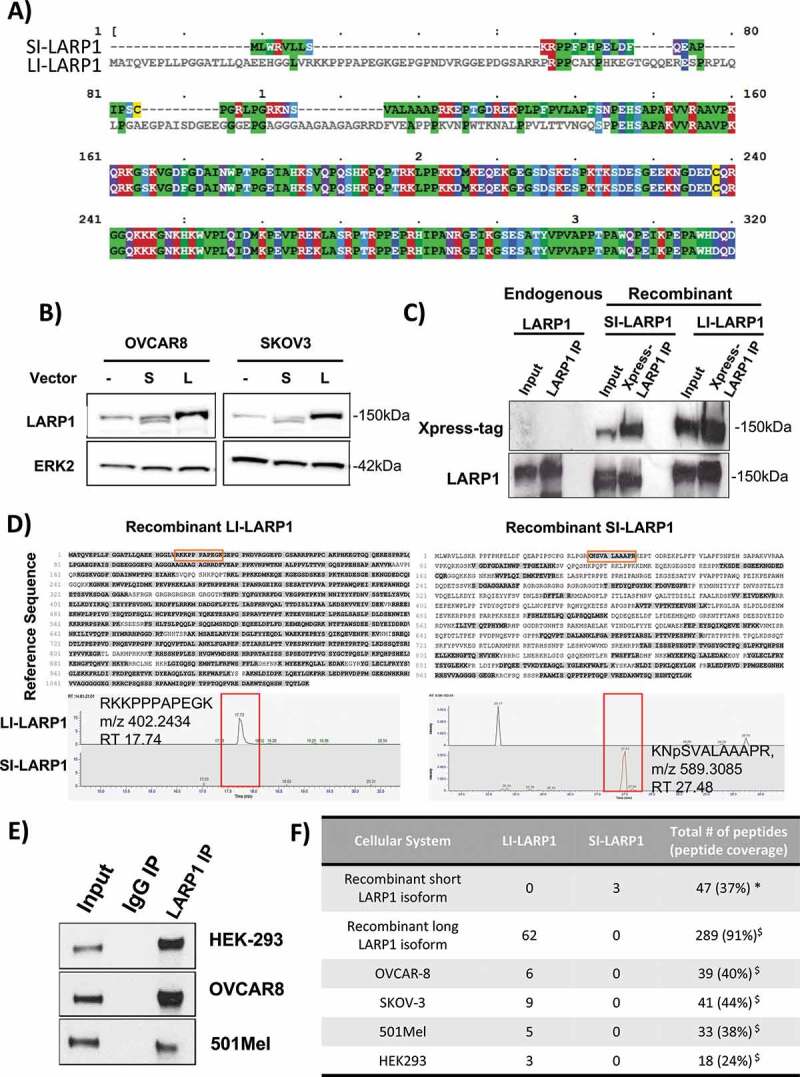
Identification of peptides specific for long isoform in four different human cell lines
A) A sequence alignment of N-terminal protein sequence of SI-and LI-LARP1 protein. B) Detection of endogenous and recombinantly expressed SI- and LI-LARP1 protein using antibody raised against peptide common to both isoforms. For expression constructs see Supporting Data 3. C) To identify the peptides corresponding to LARP1, we immunoprecipitated endogenous and overexpressed SI- and LI-LARP1 isoform using either LARP1 or Xpress tag specific antibodies. Detection of total ERK2 served as loading control. D) Immunoprecipitated samples were subjected to in solution tryptic or chemotryptic digestion and mass spectrometric analysis were performed. Resulting peptides were mapped to either long or short LARP1 protein sequence (grey shadow). Mass spectrometry identified peptides mapping specifically to short or long LARP1 isoform, when ectopically expressed (red square). MS/MS Spectra, m/z and retention time (RT) for peptides specific to SI- or LI-LARP1 are given. E) Control western blots of LARP1 pull downs using specific antibodies against LARP1. IgG control is shown. F) Summary table from peptide identification specific to LARP1 by mass spectrometry from recombinant and endogenous LARP1. Number of identified peptides specific for SI-or LI-LARP1 are given. Total number of peptides and peptide coverage for SI-(*) and LI-LARP1 ($) is given.
